PASylation of IL-1 receptor antagonist (IL-1Ra) retains IL-1 blockade and extends its duration in mouse urate crystal-induced peritonitis
- PMID: 31819009
- PMCID: PMC6970921
- DOI: 10.1074/jbc.RA119.010340
PASylation of IL-1 receptor antagonist (IL-1Ra) retains IL-1 blockade and extends its duration in mouse urate crystal-induced peritonitis
Abstract
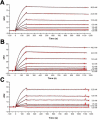
Interleukin-1 (IL-1) is a key mediator of inflammation and immunity. Naturally-occurring IL-1 receptor antagonist (IL-1Ra) binds and blocks the IL-1 receptor-1 (IL-1R1), preventing signaling. Anakinra, a recombinant form of IL-1Ra, is used to treat a spectrum of inflammatory diseases. However, anakinra is rapidly cleared from the body and requires daily administration. To create a longer-lasting alternative, PASylated IL-1Ra (PAS-IL-1Ra) has been generated by in-frame fusion of a long, defined-length, N-terminal Pro/Ala/Ser (PAS) random-coil polypeptide with IL-1Ra. Here, we compared the efficacy of two PAS-IL-1Ra molecules, PAS600-IL-1Ra and PAS800-IL-1Ra (carrying 600 and 800 PAS residues, respectively), with that of anakinra in mice. PAS600-IL-1Ra displayed markedly extended blood plasma levels 3 days post-administration, whereas anakinra was undetectable after 24 h. We also studied PAS600-IL-1Ra and PAS800-IL-1Ra for efficacy in monosodium urate (MSU) crystal-induced peritonitis. 5 days post-administration, PAS800-IL-1Ra significantly reduced leukocyte influx and inflammatory markers in MSU-induced peritonitis, whereas equimolar anakinra administered 24 h before MSU challenge was ineffective. The 6-h pretreatment with equimolar anakinra or PAS800-IL-1Ra before MSU challenge similarly reduced inflammatory markers. In cultured A549 lung carcinoma cells, anakinra, PAS600-IL-1Ra, and PAS800-IL-Ra reduced IL-1α-induced IL-6 and IL-8 levels with comparable potency. In human peripheral blood mononuclear cells, these molecules suppressed Candida albicans-induced production of the cancer-promoting cytokine IL-22. Surface plasmon resonance analyses revealed significant binding between PAS-IL-1Ra and IL-1R1, although with a slightly lower affinity than anakinra. These results validate PAS-IL-1Ra as an active IL-1 antagonist with marked in vivo potency and a significantly extended half-life compared with anakinra.
Keywords: IL-22; PASylation; biotechnology; drug delivery; half-life extension; inflammation; innate immunity; interleukin 1 (IL-1); intrinsically-disordered protein; leukocyte; pharmacokinetics.
© 2020 Powers et al.
Conflict of interest statement
C. K. E., III, and R. D. are shareholders in DNX Biopharmaceuticals, Inc., and A. S., M. S., and U. B. are shareholders of XL-protein GmbH
Figures






References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

