The human protein haptoglobin inhibits IsdH-mediated heme-sequestering by Staphylococcus aureus
- PMID: 31819010
- PMCID: PMC7029131
- DOI: 10.1074/jbc.RA119.011612
The human protein haptoglobin inhibits IsdH-mediated heme-sequestering by Staphylococcus aureus
Abstract
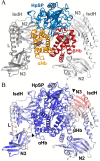
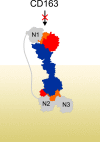
Iron is an essential nutrient for all living organisms. To acquire iron, many pathogens have developed elaborate systems to steal it from their hosts. The iron acquisition system in the opportunistic pathogen Staphylococcus aureus comprises nine proteins, called iron-regulated surface determinants (Isds). The Isd components enable S. aureus to extract heme from hemoglobin (Hb), transport it into the bacterial cytoplasm, and ultimately release iron from the porphyrin ring. IsdB and IsdH act as hemoglobin receptors and are known to actively extract heme from extracellular Hb. To limit microbial pathogenicity during infection, host organisms attempt to restrict the availability of nutrient metals at the host-pathogen interface. The human acute phase protein haptoglobin (Hp) protects the host from oxidative damage by clearing hemoglobin that has leaked from red blood cells and also restricts the availability of extracellular Hb-bound iron to invading pathogens. To investigate whether Hp serves an additional role in nutritional immunity through a direct inhibition of IsdH-mediated iron acquisition, here we measured heme extraction from the Hp-Hb complex by UV-visible spectroscopy and determined the crystal structure of the Hp-Hb-IsdH complex at 2.9 Å resolution. We found that Hp strongly inhibits IsdH-mediated heme extraction and that Hp binding prevents local unfolding of the Hb heme pocket, leaving IsdH unable to wrest the heme from Hb. Furthermore, we noted that the Hp-Hb binding appears to trap IsdH in an initial state before heme transfer. Our findings provide insights into Hp-mediated IsdH inhibition and the dynamics of IsdH-mediated heme extraction.
Keywords: Haptoglobin; Hemoglobin; IsdH; Staphylococcus aureus; Staphylococcus aureus (S. aureus); hemoglobin; infection; inhibitor; iron.
© 2020 Mikkelsen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
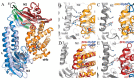
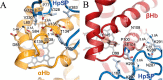


References
-
- Torres V. J., Attia A. S., Mason W. J., Hood M. I., Corbin B. D., Beasley F. C., Anderson K. L., Stauff D. L., McDonald W. H., Zimmerman L. J., Friedman D. B., Heinrichs D. E., Dunman P. M., and Skaar E. P. (2010) Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun. 78, 1618–1628 10.1128/IAI.01423-09 - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

