Metabolic response patterns in brain microdialysis fluids and serum during interstitial cisplatin treatment of high-grade glioma
- PMID: 31819184
- PMCID: PMC7052137
- DOI: 10.1038/s41416-019-0652-x
Metabolic response patterns in brain microdialysis fluids and serum during interstitial cisplatin treatment of high-grade glioma
Abstract
Background: High-grade gliomas are associated with poor prognosis. Tumour heterogeneity and invasiveness create challenges for effective treatment and use of systemically administrated drugs. Furthermore, lack of functional predictive response-assays based on drug efficacy complicates evaluation of early treatment responses.
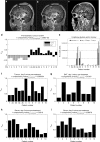
Methods: We used microdialysis to deliver cisplatin into the tumour and to monitor levels of metabolic compounds present in the tumour and non-malignant brain tissue adjacent to tumour, before and during treatment. In parallel, we collected serum samples and used multivariate statistics to analyse the metabolic effects.
Results: We found distinct metabolic patterns in the extracellular fluids from tumour compared to non-malignant brain tissue, including high concentrations of a wide range of amino acids, amino acid derivatives and reduced levels of monosaccharides and purine nucleosides. We found that locoregional cisplatin delivery had a strong metabolic effect at the tumour site, resulting in substantial release of glutamic acid, phosphate, and spermidine and a reduction of cysteine levels. In addition, patients with long-time survival displayed different treatment response patterns in both tumour and serum. Longer survival was associated with low tumour levels of lactic acid, glyceric acid, ketoses, creatinine and cysteine. Patients with longer survival displayed lower serum levels of ketohexoses, fatty acid methyl esters, glycerol-3-phosphate and alpha-tocopherol, while elevated phosphate levels were seen in both tumour and serum during treatment.
Conclusion: We highlight distinct metabolic patterns associated with high-grade tumour metabolism, and responses to cytotoxic cisplatin treatment.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Andronesi OC, Loebel F, Bogner W, Marjanska M, Vander Heiden MG, Iafrate AJ, et al. Treatment response assessment in IDH-mutant glioma patients by noninvasive 3D functional spectroscopic mapping of 2-hydroxyglutarate. Clin. Cancer Res. 2016;22:1632–1641. doi: 10.1158/1078-0432.CCR-15-0656. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

