New aspects of amino acid metabolism in cancer
- PMID: 31819187
- PMCID: PMC7052246
- DOI: 10.1038/s41416-019-0620-5
New aspects of amino acid metabolism in cancer
Abstract
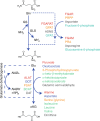
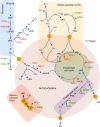
An abundant supply of amino acids is important for cancers to sustain their proliferative drive. Alongside their direct role as substrates for protein synthesis, they can have roles in energy generation, driving the synthesis of nucleosides and maintenance of cellular redox homoeostasis. As cancer cells exist within a complex and often nutrient-poor microenvironment, they sometimes exist as part of a metabolic community, forming relationships that can be both symbiotic and parasitic. Indeed, this is particularly evident in cancers that are auxotrophic for particular amino acids. This review discusses the stromal/cancer cell relationship, by using examples to illustrate a number of different ways in which cancer cells can rely on and contribute to their microenvironment - both as a stable network and in response to therapy. In addition, it examines situations when amino acid synthesis is driven through metabolic coupling to other reactions, and synthesis is in excess of the cancer cell's proliferative demand. Finally, it highlights the understudied area of non-proteinogenic amino acids in cancer metabolism and their potential role.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Sugimura K, Ohno T, Kusuyama T, Azuma I. High sensitivity of human melanoma cell lines to the growth inhibitory activity of mycoplasmal arginine deiminase in vitro. Melanoma Res. 1992;2:191–196. - PubMed
-
- Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, et al. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100:826–833.. - PubMed
-
- Gupta S, Sahu D, Bomalaski JS, Frank I, Boorjian SA, Thapa P, et al. Argininosuccinate synthetase-1 (ASS1) loss in high-grade neuroendocrine carcinomas of the urinary bladder: implications for targeted therapy with ADI-PEG 20. Endocr. Pathol. 2018;29:236–841.. - PubMed
-
- Szlosarek PW, Klabatsa A, Pallaska A, Sheaff M, Smith P, Crook T, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin. Cancer Res. 2006;12:7126–7131.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

