Carbonic anhydrase IX and acid transport in cancer
- PMID: 31819195
- PMCID: PMC7051959
- DOI: 10.1038/s41416-019-0642-z
Carbonic anhydrase IX and acid transport in cancer
Abstract
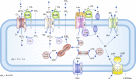
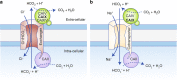
Alterations in tumour metabolism and acid/base regulation result in the formation of a hostile environment, which fosters tumour growth and metastasis. Acid/base homoeostasis in cancer cells is governed by the concerted interplay between carbonic anhydrases (CAs) and various transport proteins, which either mediate proton extrusion or the shuttling of acid/base equivalents, such as bicarbonate and lactate, across the cell membrane. Accumulating evidence suggests that some of these transporters interact both directly and functionally with CAIX to form a protein complex coined the 'transport metabolon'. Transport metabolons formed between bicarbonate transporters and CAIX require CA catalytic activity and have a function in cancer cell migration and invasion. Another type of transport metabolon is formed by CAIX and monocarboxylate transporters. In this complex, CAIX functions as a proton antenna for the transporter, which drives the export of lactate and protons from the cell. Since CAIX is almost exclusively expressed in cancer cells, these transport metabolons might serve as promising targets to interfere with tumour pH regulation and energy metabolism. This review provides an overview of the current state of research on the function of CAIX in tumour acid/base transport and discusses how CAIX transport metabolons could be exploited in modern cancer therapy.
Conflict of interest statement
The author declares no competing interests.
Figures

References
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. - PubMed
-
- Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. - PubMed
-
- Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer. 2013;13:611–623. - PubMed
-
- Vaupel P, Kallinowski F, Okunieff P. Blood-flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

