Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection
- PMID: 31819212
- PMCID: PMC6992501
- DOI: 10.1038/s41564-019-0627-y
Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection
Erratum in
-
Author Correction: Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection.Nat Microbiol. 2020 Mar;5(3):526. doi: 10.1038/s41564-020-0679-z. Nat Microbiol. 2020. PMID: 32042130
Abstract
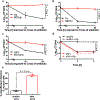
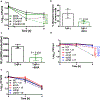
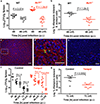
Staphylococcus aureus is a major human pathogen that causes an array of infections ranging from minor skin infections to more serious infections, including osteomyelitis, endocarditis, necrotizing pneumonia and sepsis1. These more serious infections usually arise from an initial bloodstream infection and are frequently recalcitrant to antibiotic treatment1. Phagocytosis by macrophages and neutrophils is the primary mechanism through which S. aureus infection is controlled by the immune system2. Macrophages have been shown to be a major reservoir of S. aureus in vivo3, but the role of macrophages in the induction of antibiotic tolerance has not been explored. Here, we show that macrophages not only fail to efficiently kill phagocytosed S. aureus, but also induce tolerance to multiple antibiotics. Reactive oxygen species generated by respiratory burst attack iron-sulfur cluster-containing proteins, including TCA-cycle enzymes, result in decreased respiration, lower ATP and increased antibiotic tolerance. We further show that respiratory burst induces antibiotic tolerance in the spleen during a murine systemic infection. These results suggest that a major component of the innate immune response is antagonistic to the bactericidal activities of antibiotics.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

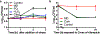







Similar articles
-
Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection.J Immunol. 2014 Apr 1;192(7):3301-7. doi: 10.4049/jimmunol.1303049. Epub 2014 Feb 21. J Immunol. 2014. PMID: 24563256 Free PMC article.
-
Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus.Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713774 Free PMC article. Review.
-
Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity.Am J Physiol Cell Physiol. 2007 Jul;293(1):C45-54. doi: 10.1152/ajpcell.00450.2006. Epub 2007 Feb 28. Am J Physiol Cell Physiol. 2007. PMID: 17329399
-
Staphylococcus aureus Lipoic Acid Synthesis Limits Macrophage Reactive Oxygen and Nitrogen Species Production To Promote Survival during Infection.Infect Immun. 2019 Sep 19;87(10):e00344-19. doi: 10.1128/IAI.00344-19. Print 2019 Oct. Infect Immun. 2019. PMID: 31308080 Free PMC article.
-
The Role of Macrophages in Staphylococcus aureus Infection.Front Immunol. 2021 Jan 19;11:620339. doi: 10.3389/fimmu.2020.620339. eCollection 2020. Front Immunol. 2021. PMID: 33542723 Free PMC article. Review.
Cited by
-
Disruption of the MreB Elongasome Is Overcome by Mutations in the Tricarboxylic Acid Cycle.Front Microbiol. 2021 Apr 23;12:664281. doi: 10.3389/fmicb.2021.664281. eCollection 2021. Front Microbiol. 2021. PMID: 33968001 Free PMC article.
-
Interruption of the tricarboxylic acid cycle in Staphylococcus aureus leads to increased tolerance to innate immunity.AIMS Microbiol. 2021 Dec 15;7(4):513-527. doi: 10.3934/microbiol.2021031. eCollection 2021. AIMS Microbiol. 2021. PMID: 35071946 Free PMC article.
-
Antibacterial Modes of Herbal Flavonoids Combat Resistant Bacteria.Front Pharmacol. 2022 Jun 27;13:873374. doi: 10.3389/fphar.2022.873374. eCollection 2022. Front Pharmacol. 2022. PMID: 35847042 Free PMC article. Review.
-
Bacterial metabolism and susceptibility to cell wall-active antibiotics.Adv Microb Physiol. 2023;83:181-219. doi: 10.1016/bs.ampbs.2023.04.002. Epub 2023 May 16. Adv Microb Physiol. 2023. PMID: 37507159 Free PMC article. Review.
-
Genetic Determinants of Intrinsic Antibiotic Tolerance in Mycobacterium avium.Microbiol Spectr. 2021 Oct 31;9(2):e0024621. doi: 10.1128/Spectrum.00246-21. Epub 2021 Sep 15. Microbiol Spectr. 2021. PMID: 34523947 Free PMC article.
References
-
- Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM & Van Bambeke F Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob AgentsChemother 50, 841–851, doi:10.1128/AAC.50.3.841-851.2006 (2006). - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

