Towards common ground in the biodiversity-disease debate
- PMID: 31819238
- PMCID: PMC7224049
- DOI: 10.1038/s41559-019-1060-6
Towards common ground in the biodiversity-disease debate
Abstract
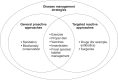
The disease ecology community has struggled to come to consensus on whether biodiversity reduces or increases infectious disease risk, a question that directly affects policy decisions for biodiversity conservation and public health. Here, we summarize the primary points of contention regarding biodiversity-disease relationships and suggest that vector-borne, generalist wildlife and zoonotic pathogens are the types of parasites most likely to be affected by changes to biodiversity. One synthesis on this topic revealed a positive correlation between biodiversity and human disease burden across countries, but as biodiversity changed over time within these countries, this correlation became weaker and more variable. Another synthesis-a meta-analysis of generally smaller-scale experimental and field studies-revealed a negative correlation between biodiversity and infectious diseases (a dilution effect) in various host taxa. These results raise the question of whether biodiversity-disease relationships are more negative at smaller spatial scales. If so, biodiversity conservation at the appropriate scales might prevent wildlife and zoonotic diseases from increasing in prevalence or becoming problematic (general proactive approaches). Further, protecting natural areas from human incursion should reduce zoonotic disease spillover. By contrast, for some infectious diseases, managing particular species or habitats and targeted biomedical approaches (targeted reactive approaches) might outperform biodiversity conservation as a tool for disease control. Importantly, biodiversity conservation and management need to be considered alongside other disease management options. These suggested guiding principles should provide common ground that can enhance scientific and policy clarity for those interested in simultaneously improving wildlife and human health.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. - PubMed
-
- Rohr JR, Bernhardt ES, Cadotte MW, Clements WH. The ecology and economics of restoration: when, what, where, and how to restore ecosystems. Ecol. Soc. 2018;23:15.
-
- Dornelas M, et al. Assemblage time series reveal biodiversity change but not systematic loss. Science. 2014;344:296–299. - PubMed
-
- Elton, C. S. The Ecology of Invasions by Animals and Plants (Methuen Publishing, 1958).
-
- Van der Plank, J. E. Plant Diseases: Epidemics and Control (Academic Press, 1963).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

