Phenotypic Properties of Collagen in Dentinogenesis Imperfecta Associated with Osteogenesis Imperfecta
- PMID: 31819441
- PMCID: PMC6897053
- DOI: 10.2147/IJN.S217420
Phenotypic Properties of Collagen in Dentinogenesis Imperfecta Associated with Osteogenesis Imperfecta
Abstract
Introduction: Dentinogenesis imperfecta type 1 (OIDI) is considered a relatively rare genetic disorder (1:5000 to 1:45,000) associated with osteogenesis imperfecta. OIDI impacts the formation of collagen fibrils in dentin, leading to morphological and structural changes that affect the strength and appearance of teeth. However, there is still a lack of understanding regarding the nanoscale characterization of the disease, in terms of collagen ultrastructure and mechanical properties. Therefore, this research presents a qualitative and quantitative report into the phenotype and characterization of OIDI in dentin, by using a combination of imaging, nanomechanical approaches.
Methods: For this study, 8 primary molars from OIDI patients and 8 primary control molars were collected, embedded in acrylic resin and cut into longitudinal sections. Sections were then demineralized in 37% phosphoric acid using a protocol developed in-house. Initial experiments demonstrated the effectiveness of the demineralization protocol, as the ATR-FTIR spectral fingerprints showed an increase in the amide bands together with a decrease in phosphate content. Structural and mechanical analyses were performed directly on both the mineralized and demineralized samples using a combination of scanning electron microscopy, atomic force microscopy, and Wallace indentation.
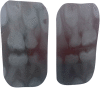
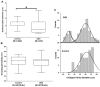
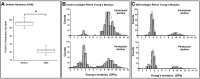
Results: Mesoscale imaging showed alterations in dentinal tubule morphology in OIDI patients, with a reduced number of tubules and a decreased tubule diameter compared to healthy controls. Nanoscale collagen ultrastructure presented a similar D-banding periodicity between OIDI and controls. Reduced collagen fibrils diameter was also recorded for the OIDI group. The hardness of the (mineralized) control dentin was found to be significantly higher (p<0.05) than that of the OIDI (mineralized) dentine. Both the exposed peri- and intratubular dentinal collagen presented bimodal elastic behaviors (Young's moduli). The control samples presented a stiffening of the intratubular collagen when compared to the peritubular collagen. In case of the OIDI, this stiffening in the collagen between peri- and intratubular dentinal collagen was not observed and the exposed collagen presented overall a lower elasticity than the control samples.
Conclusion: This study presents a systematic approach to the characterization of collagen structure and properties in OIDI as diagnosed in dentin. Structural markers for OIDI at the mesoscale and nanoscale were found and correlated with an observed lack of increased elastic moduli of the collagen fibrils in the intratubular OIDI dentin. These findings offer an explanation of how structural changes in the dentin could be responsible for the failure of some adhesive restorative materials as observed in patients affected by OIDI.
Keywords: atomic force microscopy; collagen; demineralisation; dentin; dentinogenesis imperfecta; dentistry.
© 2019 Ibrahim et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Witkop CJ, Jr. Hereditary defects of dentin. Dent Clin North Am. 1975;19:25–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

