LncRNA NEAT1 Promotes Proliferation, Migration And Invasion Via Regulating miR-296-5p/CNN2 Axis In Hepatocellular Carcinoma Cells
- PMID: 31819486
- PMCID: PMC6874127
- DOI: 10.2147/OTT.S228917
LncRNA NEAT1 Promotes Proliferation, Migration And Invasion Via Regulating miR-296-5p/CNN2 Axis In Hepatocellular Carcinoma Cells
Retraction in
-
LncRNA NEAT1 Promotes Proliferation, Migration and Invasion via Regulating miR-296-5p/CNN2 Axis in Hepatocellular Carcinoma Cells [Retraction].Onco Targets Ther. 2021 Oct 21;14:5095-5096. doi: 10.2147/OTT.S344971. eCollection 2021. Onco Targets Ther. 2021. PMID: 34707367 Free PMC article.
Abstract
Background: Emerging evidence has revealed that long noncoding RNA nuclear paraspeckle assembly transcript 1 (lncRNA NEAT1) is implicated in the development of various cancers. However, the underlying molecular mechanisms of NEAT1 in hepatocellular carcinoma (HCC) remain unclear.
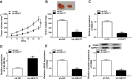
Methods: The expression of NEAT1, miR-296-5p and Calponin 2 (CNN2) was detected by quantitative real-time polymerase chain reaction or Western blot, respectively. Cell proliferation and apoptosis were analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay or flow cytometry, respectively. Transwell assay was used to determine cell migration and invasion. The interaction between miR-296-5p and NEAT1 or CNN2 was analyzed by dual-luciferase reporter assay and RIP assay. Huh7 cells transfected with sh-NEAT1 were used to establish the murine xenograft model.
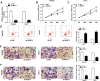
Results: NEAT1 was elevated in HCC tissues and cell lines. Knockdown of NEAT1 significantly inhibited proliferation, migration and invasion of HCC cells in vitro as well as tumor growth in vivo. NEAT1 was a sponge of miR-296-5p and remarkably reduced the level of miR-296-5p in HCC cells. Furthermore, NEAT1 silence significantly decreased the expression of CNN2, which was the direct target of miR-296-5p. Besides that, the tumor suppression caused by NEAT1 silence could be rescued by CNN2 restoration or miR-296-5p inhibition in vitro. Additionally, NEAT1 indirectly regulated CNN2 expression by competing to miR-296-5p in vitro and in vivo.
Conclusion: LncRNA NEAT1 contributes to HCC progression by regulating miR-296-5p/CNN2 axis, providing a novel regulatory mechanism for HCC development and a promising therapeutic target for the HCC treatment.
Keywords: CNN2; HCC; NEAT1; miR-296-5p; progression.
© 2019 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




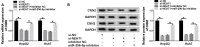
References
-
- Stotz M, Gerger A, Haybaeck J, et al. Molecular targeted therapies in hepatocellular carcinoma: past, present and future. Anticancer Res. 2015;35(11):5737–5744. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

