Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells
- PMID: 31820335
- PMCID: PMC7272517
- DOI: 10.1007/s12079-019-00539-1
Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells
Abstract
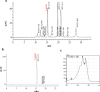
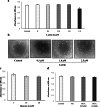
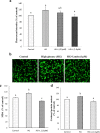
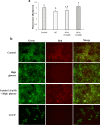
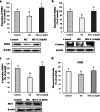
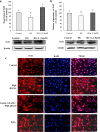
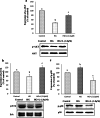
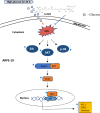
Diabetic retinopathy (DR) is a major cause of acquired blindness among working adults. The retinal pigment epithelium (RPE), constitutes an outer blood-retinal barrier, is vastly affected in diabetic humans and animals. Lower levels of lutein in the serum and retina of diabetic population, and beneficial effects of carotenoids supplementation in diabetic retinopathy patients created an interest to examine the protective effect of lutein on hyperglycemia-mediated changes in oxidative stress and antioxidant defense system in ARPE-19 cells. The WST-1 assay was performed to analyze the impact of glucose, and lutein on the viability of ARPE-19. The intracellular oxidative stress was measured by a DCF (dichlorofluorescein) assay, mitochondrial membrane potential (MMP) was monitored using a JC-10 MMP assay kit and GSH level was examined using GSH/GSSG ratio detection kit. The oxidative stress markers, protein carbonyl and malondialdehyde were spectrophotometrically measured using 2,4-dinitrophenylhydrazine and 2-thiobarbituric acid, respectively. The expression of endogenous antioxidant enzymes and regulatory proteins in ARPE-19 was quantified by western blotting. The localization of Nrf2 protein was examined by immunofluorescent staining. The results show that lutein (up to 1.0 μM) did not affect the viability of ARPE-19 grown in both normal and high-glucose conditions. Lutein treatment blocked high glucose-mediated elevation of intracellular ROS, protein carbonyl and malondialdehyde content in ARPE-19 cells. The decreased MMP and GSH levels observed in ARPE-19 grown under high-glucose condition were rescued by lutein treatment. Further, lutein protected high glucose-mediated down-regulation of a redox-sensitive transcription factor, Nrf2, and antioxidant enzymes, SOD2, HO-1, and catalase. This protective effect of lutein was linked with activated nuclear translocation of Nrf2, which was associated with increased activation of regulatory proteins such as Erk and AKT. Our study indicates that improving the concentration of lutein in the retina could protect RPE from diabetes-associated damage.
Keywords: ARPE-19; Hyperglycemia; Lutein; ROS; Redox signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. - DOI - PubMed
-
- Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, Zick A, Sharoni Y, Levy J. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4:177–186. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

