A novel method to improve the osteogenesis capacity of hUCMSCs with dual-directional pre-induction under screened co-culture conditions
- PMID: 31820506
- PMCID: PMC7078770
- DOI: 10.1111/cpr.12740
A novel method to improve the osteogenesis capacity of hUCMSCs with dual-directional pre-induction under screened co-culture conditions
Erratum in
-
Correction.Cell Prolif. 2024 Feb;57(2):e13572. doi: 10.1111/cpr.13572. Epub 2024 Jan 3. Cell Prolif. 2024. PMID: 38173075 Free PMC article. No abstract available.
Abstract
Objectives: Mesenchymal stem cells (MSCs) based therapy for bone regeneration has been regarded as a promising method in the clinic. However, hBMSCs with invasive harvesting process and undesirable proliferation rate hinder the extensive usage. HUCMSCs of easier access and excellent performances provide an alternative for the fabrication of tissue-engineered bone construct. Evidence suggested the osteogenesis ability of hUCMSCs was weaker than that of hBMSCs. To address this issue, a co-culture strategy of osteogenically and angiogenically induced hUCMSCs has been proposed since thorough vascularization facilitates the blood-borne nutrition and oxygen to transport in the scaffold, synergistically expediting the process of ossification.
Materials and methods: Herein, we used osteogenic- and angiogenic-differentiated hUCMSCs for co-culture in screened culture medium to elevate the osteogenic capacity with in vitro studies and finally coupled with 3D TCP scaffold to repair rat's critical-sized calvarial bone defect. By dual-directional induction, hUCMSCs could differentiate into osteoblasts and endothelial cells, respectively. To optimize the co-culture condition, gradient ratios of dual-directional differentiated hUCMSCs co-cultured under different medium were studied to determine the appropriate condition.
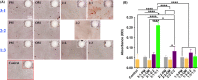
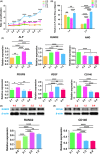
Results: It revealed that the osteogenic- and angiogenic-induced hUCMSCs mixed with the ratio of 3:1 co-cultured in the mixed medium of osteogenic induction medium to endothelial cell induction medium of 3:1 possessed more mineralization nodules. Similarly, ALP and osteogenesis/angiogenesis-related genes expressions were relatively higher. Further evidence of bone defect repair with 3D printed TCP of 3:1 group exhibited better restoration outcomes.
Conclusions: Our work demonstrated a favourable and convenient approach of dual-directional differentiated hUCMSCs co-culture to improve the osteogenesis, establishing a novel way to fabricate tissue-engineered bone graft with 3D TCP for large bone defect augmentation.
Keywords: bone defect; co-culture; dual-directional differentiation; human umbilical cord mesenchymal stem cells; osteogenesis.
© 2019 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures




Similar articles
-
Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs.J Tissue Eng Regen Med. 2018 Jan;12(1):191-203. doi: 10.1002/term.2395. Epub 2017 Jun 13. J Tissue Eng Regen Med. 2018. PMID: 28098961 Free PMC article.
-
The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells.PLoS One. 2015 Mar 23;10(3):e0120593. doi: 10.1371/journal.pone.0120593. eCollection 2015. PLoS One. 2015. PMID: 25799169 Free PMC article.
-
Enhanced bone tissue regeneration of a biomimetic cellular scaffold with co-cultured MSCs-derived osteogenic and angiogenic cells.Cell Prolif. 2019 Sep;52(5):e12658. doi: 10.1111/cpr.12658. Epub 2019 Jul 11. Cell Prolif. 2019. PMID: 31297910 Free PMC article.
-
Research Progress on the Osteogenesis-Related Regulatory Mechanisms of Human Umbilical Cord Mesenchymal Stem Cells.Stem Cell Rev Rep. 2023 Jul;19(5):1252-1267. doi: 10.1007/s12015-023-10521-5. Epub 2023 Mar 14. Stem Cell Rev Rep. 2023. PMID: 36917312 Review.
-
Overcoming physical constraints in bone engineering: 'the importance of being vascularized'.J Biomater Appl. 2016 Feb;30(7):940-51. doi: 10.1177/0885328215616749. Epub 2015 Dec 1. J Biomater Appl. 2016. PMID: 26637441 Review.
Cited by
-
Osteogenically committed hUCMSCs-derived exosomes promote the recovery of critical-sized bone defects with enhanced osteogenic properties.APL Bioeng. 2024 Feb 6;8(1):016107. doi: 10.1063/5.0159740. eCollection 2024 Mar. APL Bioeng. 2024. PMID: 38327715 Free PMC article.
-
β-TCP from 3D-printed composite scaffolds acts as an effective phosphate source during osteogenic differentiation of human mesenchymal stromal cells.Front Cell Dev Biol. 2023 Oct 26;11:1258161. doi: 10.3389/fcell.2023.1258161. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37965582 Free PMC article.
-
Stem cell therapy: old challenges and new solutions.Mol Biol Rep. 2020 Apr;47(4):3117-3131. doi: 10.1007/s11033-020-05353-2. Epub 2020 Mar 3. Mol Biol Rep. 2020. PMID: 32128709 Review.
-
Co-Culture Approaches in Cartilage and Bone Tissue Regeneration.Int J Mol Sci. 2025 Jun 14;26(12):5711. doi: 10.3390/ijms26125711. Int J Mol Sci. 2025. PMID: 40565176 Free PMC article. Review.
-
Use of stem cells in bone regeneration in cleft palate patients: review and recommendations.J Korean Assoc Oral Maxillofac Surg. 2022 Apr 30;48(2):71-78. doi: 10.5125/jkaoms.2022.48.2.71. J Korean Assoc Oral Maxillofac Surg. 2022. PMID: 35491137 Free PMC article. Review.
References
-
- Mueller SM, Glowacki J. Age‐related decline in the osteogenic potential of human bone marrow cells cultured in three‐dimensional collagen sponges. J Cell Biochem. 2001;82:583‐590. - PubMed
MeSH terms
Grants and funding
- 2017FE468-168/Joint Fund for Applied Basic Research of Yunnan Provincial Science and Technology Department-Kunming Medical School
- 881_2012/International Team for Implantology
- 2016YFC1102900/National Major Science and Technology Project of China
- 81671029/National Nature Science Foundation of China
- 201704030024/Guangzhou Science, Technology and Innovation Commission
LinkOut - more resources
Full Text Sources

