SNX27-retromer assembly recycles MT1-MMP to invadopodia and promotes breast cancer metastasis
- PMID: 31820782
- PMCID: PMC7039210
- DOI: 10.1083/jcb.201812098
SNX27-retromer assembly recycles MT1-MMP to invadopodia and promotes breast cancer metastasis
Abstract
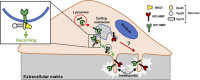
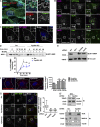
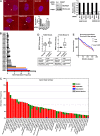
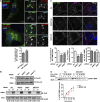
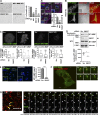
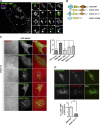
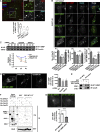
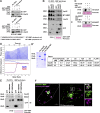
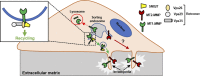
A variety of metastatic cancer cells use actin-rich membrane protrusions, known as invadopodia, for efficient ECM degradation, which involves trafficking of proteases from intracellular compartments to these structures. Here, we demonstrate that in the metastatic breast cancer cell line MDA-MB-231, retromer regulates the matrix invasion activity by recycling matrix metalloprotease, MT1-MMP. We further found that MT2-MMP, another abundantly expressed metalloprotease, is also invadopodia associated. MT1- and MT2-MMP showed a high degree of colocalization but were located on the distinct endosomal domains. Retromer and its associated sorting nexin, SNX27, phenocopied each other in matrix degradation via selectively recycling MT1-MMP but not MT2-MMP. ITC-based studies revealed that both SNX27 and retromer could directly interact with MT1-MMP. Analysis from a publicly available database showed SNX27 to be overexpressed or frequently altered in the patients having invasive breast cancer. In xenograft-based studies, SNX27-depleted cell lines showed prolonged survival of SCID mice, suggesting a possible implication for overexpression of the sorting nexin in tumor samples.
© 2019 Sharma et al.
Figures

Similar articles
-
DLC3 suppresses MT1-MMP-dependent matrix degradation by controlling RhoB and actin remodeling at endosomal membranes.J Cell Sci. 2019 Jun 5;132(11):jcs223172. doi: 10.1242/jcs.223172. J Cell Sci. 2019. PMID: 31076513
-
CRP2, a new invadopodia actin bundling factor critically promotes breast cancer cell invasion and metastasis.Oncotarget. 2016 Mar 22;7(12):13688-705. doi: 10.18632/oncotarget.7327. Oncotarget. 2016. PMID: 26883198 Free PMC article.
-
Sorting nexin 9 negatively regulates invadopodia formation and function in cancer cells.J Cell Sci. 2016 Jul 15;129(14):2804-16. doi: 10.1242/jcs.188045. Epub 2016 Jun 8. J Cell Sci. 2016. PMID: 27278018 Free PMC article.
-
Cellular and Molecular Mechanisms of MT1-MMP-Dependent Cancer Cell Invasion.Annu Rev Cell Dev Biol. 2016 Oct 6;32:555-576. doi: 10.1146/annurev-cellbio-111315-125227. Epub 2016 Aug 8. Annu Rev Cell Dev Biol. 2016. PMID: 27501444 Review.
-
Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia.J Cell Sci. 2009 Sep 1;122(Pt 17):3015-24. doi: 10.1242/jcs.034561. J Cell Sci. 2009. PMID: 19692588 Review.
Cited by
-
SNX5 suppresses clear cell renal cell carcinoma progression by inducing CD44 internalization and epithelial-to-mesenchymal transition.Mol Ther Oncolytics. 2021 Dec 6;24:87-100. doi: 10.1016/j.omto.2021.12.002. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2021. PMID: 35024436 Free PMC article.
-
Elevated HNF1A expression promotes radiation-resistance via driving PI3K/AKT signaling pathway in esophageal squamous cell carcinoma cells.J Cancer. 2021 Jun 16;12(16):5013-5024. doi: 10.7150/jca.58023. eCollection 2021. J Cancer. 2021. PMID: 34234870 Free PMC article.
-
Monitoring Endosomal Cargo Retrieval to the Trans-Golgi Network by Microscopic and Biochemical Approaches.Methods Mol Biol. 2022;2473:213-236. doi: 10.1007/978-1-0716-2209-4_16. Methods Mol Biol. 2022. PMID: 35819769
-
Insights into cargo sorting by SNX32 and its role in neurite outgrowth.Elife. 2023 May 9;12:e84396. doi: 10.7554/eLife.84396. Elife. 2023. PMID: 37158588 Free PMC article.
-
Adaptations of membrane trafficking in cancer and tumorigenesis.J Cell Sci. 2024 May 15;137(10):jcs260943. doi: 10.1242/jcs.260943. Epub 2024 May 21. J Cell Sci. 2024. PMID: 38770683 Free PMC article. Review.
References
-
- Artym V.V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K.M., and Mueller S.C.. 2006. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66:3034–3043. 10.1158/0008-5472.CAN-05-2177 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

