Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome
- PMID: 31820802
- PMCID: PMC6936723
- DOI: 10.1093/humrep/dez205
Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome
Abstract
Study question: Can kisspeptin treatment induce gonadotrophin responses and ovulation in preclinical models and anovulatory women with polycystic ovary syndrome (PCOS)?
Summary answer: Kisspeptin administration in some anovulatory preclinical models and women with PCOS can stimulate reproductive hormone secretion and ovulation, albeit with incomplete efficacy.
What is known already: PCOS is a prevalent, heterogeneous endocrine disorder, characterized by ovulatory dysfunction, hyperandrogenism and deregulated gonadotrophin secretion, in need of improved therapeutic options. Kisspeptins (encoded by Kiss1) are master regulators of the reproductive axis, acting mainly at GnRH neurons, with kisspeptins being an essential drive for gonadotrophin-driven ovarian follicular maturation and ovulation. Altered Kiss1 expression has been found in rodent models of PCOS, although the eventual pathophysiological role of kisspeptins in PCOS remains unknown.
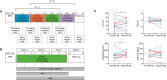
Study design, size, duration: Gonadotrophin and ovarian/ovulatory responses to kisspeptin-54 (KP-54) were evaluated in three preclinical models of PCOS, generated by androgen exposures at different developmental windows, and a pilot exploratory cohort of anovulatory women with PCOS.
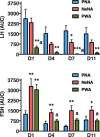
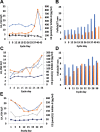
Participants/materials, setting, methods: Three models of PCOS were generated by exposure of female rats to androgens at different periods of development: PNA (prenatal androgenization; N = 20), NeNA (neonatal androgenization; N = 20) and PWA (post-weaning androgenization; N = 20). At adulthood (postnatal day 100), rats were subjected to daily treatments with a bolus of KP-54 (100 μg/kg, s.c.) or vehicle for 11 days (N = 10 per model and treatment). On Days 1, 4, 7 and 11, LH and FSH responses were assessed at different time-points within 4 h after KP-54 injection, while ovarian responses, in terms of follicular maturation and ovulation, were measured at the end of the treatment. In addition, hormonal (gonadotrophin, estrogen and inhibin B) and ovulatory responses to repeated KP-54 administration, at doses of 6.4-12.8 nmol/kg, s.c. bd for 21 days, were evaluated in a pilot cohort of anovulatory women (N = 12) diagnosed with PCOS, according to the Rotterdam criteria.
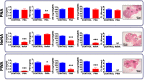
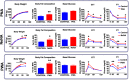
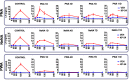
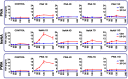
Main results and the role of chance: Deregulated reproductive indices were detected in all PCOS models: PNA, NeNA and PWA. Yet, anovulation was observed only in NeNA and PWA rats. However, while anovulatory NeNA rats displayed significant LH and FSH responses to KP-54 (P < 0.05), which rescued ovulation, PWA rats showed blunted LH secretion after repeated KP-54 injection and failed to ovulate. In women with PCOS, KP-54 resulted in a small rise in LH (P < 0.05), with an equivalent elevation in serum estradiol levels (P < 0.05). Two women showed growth of a dominant follicle with subsequent ovulation, one woman displayed follicle growth but not ovulation and desensitization was observed in another patient. No follicular response was detected in the other women.
Limitations, reasons for caution: While three different preclinical PCOS models were used in order to capture the heterogeneity of clinical presentations of the syndrome, it must be noted that rat models recapitulate many but not all the features of this condition. Additionally, our pilot study was intended as proof of principle, and the number of participants is low, but the convergent findings in preclinical and clinical studies reinforce the validity of our conclusions.
Wider implications of the findings: Our first-in-rodent and -human studies demonstrate that KP-54 administration in anovulatory preclinical models and women with PCOS can stimulate reproductive hormone secretion and ovulation, albeit with incomplete efficacy. As our rat models likely reflect the diversity of PCOS phenotypes, our results argue for the need of personalized management of anovulatory dysfunction in women with PCOS, some of whom may benefit from kisspeptin-based treatments.
Study funding/competing interest(s): This work was supported by research agreements between Ferring Research Institute and the Universities of Cordoba and Edinburgh. K.S. was supported by the Wellcome Trust Scottish Translational Medicine and Therapeutics Initiative (STMTI). Some of this work was undertaken in the MRC Centre for Reproductive Health which is funded by the MRC Centre grant MR/N022556/1. M.T.-S. is a member of CIBER Fisiopatología de la Obesidad y Nutrición, which is an initiative of Instituto de Salud Carlos III. Dr Mannaerts is an employee of Ferring International PharmaScience Center (Copenhagen, Denmark), and Drs Qi, van Duin and Kohout are employees of the Ferring Research Institute (San Diego, USA). Dr Anderson and Dr Tena-Sempere were recipients of a grant support from the Ferring Research Institute, and Dr Anderson has undertaken consultancy work and received speaker fees outside this study from Merck, IBSA, Roche Diagnostics, NeRRe Therapeutics and Sojournix Inc. Dr Skorupskaite was supported by the Wellcome Trust through the Scottish Translational Medicine and Therapeutics Initiative 102419/Z/13/A. The other authors have no competing interest.
Keywords: gonadotrophins; kisspeptin; ovarian stimulation; ovulation; polycystic ovary syndrome; preclinical models.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures

Similar articles
-
Kisspeptin and neurokinin B interactions in modulating gonadotropin secretion in women with polycystic ovary syndrome.Hum Reprod. 2020 Jun 1;35(6):1421-1431. doi: 10.1093/humrep/deaa104. Hum Reprod. 2020. PMID: 32510130 Free PMC article. Clinical Trial.
-
Selective loss of kisspeptin signaling in oocytes causes progressive premature ovulatory failure.Hum Reprod. 2022 Apr 1;37(4):806-821. doi: 10.1093/humrep/deab287. Hum Reprod. 2022. PMID: 35037941 Free PMC article.
-
Targeted inhibition of kisspeptin neurons reverses hyperandrogenemia and abnormal hyperactive LH secretion in a preclinical mouse model of polycystic ovary syndrome.Hum Reprod. 2024 Sep 1;39(9):2089-2103. doi: 10.1093/humrep/deae153. Hum Reprod. 2024. PMID: 38978296 Free PMC article.
-
The role of kisspeptin in the pathogenesis of a polycystic ovary syndrome.Endocr Regul. 2023 Dec 21;57(1):292-303. doi: 10.2478/enr-2023-0032. Print 2023 Jan 1. Endocr Regul. 2023. PMID: 38127687 Review.
-
The step-down principle in gonadotrophin treatment and the role of GnRH analogues.Baillieres Clin Obstet Gynaecol. 1993 Jun;7(2):309-30. doi: 10.1016/s0950-3552(05)80133-6. Baillieres Clin Obstet Gynaecol. 1993. PMID: 8358893 Review.
Cited by
-
Early programming of reproductive health and fertility: novel neuroendocrine mechanisms and implications in reproductive medicine.Hum Reprod Update. 2022 May 2;28(3):346-375. doi: 10.1093/humupd/dmac005. Hum Reprod Update. 2022. PMID: 35187579 Free PMC article. Review.
-
Polycystic ovary syndrome: pathophysiology and therapeutic opportunities.BMJ Med. 2023 Oct 12;2(1):e000548. doi: 10.1136/bmjmed-2023-000548. eCollection 2023. BMJ Med. 2023. PMID: 37859784 Free PMC article. Review.
-
Serum kisspeptin as a promising biomarker for PCOS: a mini review of current evidence and future prospects.Clin Diabetes Endocrinol. 2024 Sep 30;10(1):27. doi: 10.1186/s40842-024-00190-9. Clin Diabetes Endocrinol. 2024. PMID: 39343941 Free PMC article. Review.
-
The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction.Front Endocrinol (Lausanne). 2022 Jun 28;13:925206. doi: 10.3389/fendo.2022.925206. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35837314 Free PMC article. Review.
-
Advances in clinical applications of kisspeptin-GnRH pathway in female reproduction.Reprod Biol Endocrinol. 2022 May 23;20(1):81. doi: 10.1186/s12958-022-00953-y. Reprod Biol Endocrinol. 2022. PMID: 35606759 Free PMC article. Review.
References
-
- Abbara A, Clarke S, Islam R, Prague JK, Comninos AN, Narayanaswamy S, Papadopoulou D, Roberts R, Izzi-Engbeaya C, Ratnasabapathy R et al. . A second dose of kisspeptin-54 improves oocyte maturation in women at high risk of ovarian hyperstimulation syndrome: a phase 2 randomized controlled trial. Hum Reprod 2017;32:1915–1924. - PMC - PubMed
-
- Abbara A, Jayasena CN, Christopoulos G, Narayanaswamy S, Izzi-Engbeaya C, Nijher GM, Comninos AN, Peters D, Buckley A, Ratnasabapathy R et al. . Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J Clin Endocrinol Metab 2015;100:3322–3331. - PMC - PubMed
-
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 2005;11:357–374. - PubMed
-
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995;10:2107–2111. - PubMed
-
- Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 2006;12:351–361. - PubMed

