Pancreatic alpha-cell mass across adult human lifespan
- PMID: 31821160
- PMCID: PMC6944979
- DOI: 10.1530/EJE-19-0844
Pancreatic alpha-cell mass across adult human lifespan
Abstract
Aim: To establish pancreatic alpha-cell mass in lean, non-diabetic humans over the adult lifespan, performed as a follow-up study to beta-cell mass across the adult human lifespan.
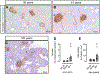
Methods: We examined human pancreatic autopsy tissue from 66 lean, non-diabetic individuals aged from 30 to 102 years, grouped into deciles: 3rd (30-39 years), 4th (40-49 years), 5th (50-59 years), 6th (60-69 years), 7th (70-79 years), 8th (80-89 years) and 9th deciles (90+ years). Sections of pancreas were immunostained for glucagon and analyzed for fractional alpha-cell area. Population-based pancreatic volume data were used to calculate alpha-cell mass.
Results: With advanced age, the exocrine pancreas undergoes atrophy demonstrated by increased fat area (as % exocrine area) (0.05 ± 0.01 vs 1.6 ± 0.7% fat area of total exocrine pancreas, 3rd vs 9th decile, P < 0.05). Consequently, islet density increases with age (2.7 ± 0.4 vs 10.5 ± 3.3 islets/mm2, 3rd vs 9th decile, P < 0.05). Alpha-cell fractional area increases with advanced age (0.34 ± 0.05% vs 0.73 ± 0.26%, 3rd vs 9th decile, P < 0.05). However, alpha-cell mass remains constant at ~190 mg throughout the adult lifespan in lean, non-diabetic humans. Within islets, alpha-cell distribution between mantle and core is unchanged across deciles (1862 ± 220 vs 1945 ± 200 vs 1948 ± 139 alpha cells in islet mantle/mm2, 3rd vs 6th vs 9th decile, P = 0.93 and 1912 ± 442 vs 1449 ± 123 vs 1514 ± 168 alpha cells in islet core/mm2, 3rd vs 6th vs 9th decile, P = 0.47), suggesting that human islets retain their structural organization in the setting of age-related exocrine atrophy.
Conclusions: Consistent with our previous findings for beta-cell mass, alpha-cell mass remains constant in humans, even with advanced age. Pancreatic endocrine cells are much more robustly preserved than exocrine cells in aged humans, and islets maintain their structural integrity throughout life.
Conflict of interest statement
CONFLICT OF INTERESTS
There is no conflict of interests.
Figures
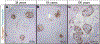

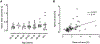


References
-
- Dunning BE, Foley JE & Ahren B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia 2005. 48 1700–1713. - PubMed
-
- Unger RH & Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975. 1 14–16. - PubMed
-
- Unger RH. Glucagon and insulin: a bihormonal system. Compr Ther 1976. 2 20–26. - PubMed

