Replacing murine insulin 1 with human insulin protects NOD mice from diabetes
- PMID: 31821343
- PMCID: PMC6903741
- DOI: 10.1371/journal.pone.0225021
Replacing murine insulin 1 with human insulin protects NOD mice from diabetes
Abstract
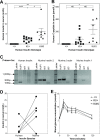
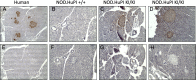
Type 1, or autoimmune, diabetes is caused by the T-cell mediated destruction of the insulin-producing pancreatic beta cells. Non-obese diabetic (NOD) mice spontaneously develop autoimmune diabetes akin to human type 1 diabetes. For this reason, the NOD mouse has been the preeminent murine model for human type 1 diabetes research for several decades. However, humanized mouse models are highly sought after because they offer both the experimental tractability of a mouse model and the clinical relevance of human-based research. Autoimmune T-cell responses against insulin, and its precursor proinsulin, play central roles in the autoimmune responses against pancreatic beta cells in both humans and NOD mice. As a first step towards developing a murine model of the human autoimmune response against pancreatic beta cells we set out to replace the murine insulin 1 gene (Ins1) with the human insulin gene (Ins) using CRISPR/Cas9. Here we describe a NOD mouse strain that expresses human insulin in place of murine insulin 1, referred to as HuPI. HuPI mice express human insulin, and C-peptide, in their serum and pancreata and have normal glucose tolerance. Compared with wild type NOD mice, the incidence of diabetes is much lower in HuPI mice. Only 15-20% of HuPI mice developed diabetes after 300 days, compared to more than 60% of unmodified NOD mice. Immune-cell infiltration into the pancreatic islets of HuPI mice was not detectable at 100 days but was clearly evident by 300 days. This work highlights the feasibility of using CRISPR/Cas9 to create mouse models of human diseases that express proteins pivotal to the human disease. Furthermore, it reveals that even subtle changes in proinsulin protect NOD mice from diabetes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Investigation of type 1 diabetes in NOD mice knockout for the osteopontin gene.Gene. 2020 Aug 30;753:144785. doi: 10.1016/j.gene.2020.144785. Epub 2020 May 20. Gene. 2020. PMID: 32445922
-
Insulin-producing intestinal K cells protect nonobese diabetic mice from autoimmune diabetes.Gastroenterology. 2014 Jul;147(1):162-171.e6. doi: 10.1053/j.gastro.2014.03.020. Epub 2014 Mar 21. Gastroenterology. 2014. PMID: 24662331
-
Pancreatic Alpha-Cells Contribute Together With Beta-Cells to CXCL10 Expression in Type 1 Diabetes.Front Endocrinol (Lausanne). 2020 Sep 15;11:630. doi: 10.3389/fendo.2020.00630. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33042009 Free PMC article.
-
Type I diabetes mellitus: a predictable autoimmune disease with interindividual variation in the rate of beta cell destruction.Clin Immunol Immunopathol. 1989 Jan;50(1 Pt 2):S85-95. doi: 10.1016/0090-1229(89)90115-3. Clin Immunol Immunopathol. 1989. PMID: 2642771 Review.
-
Genetic and immunological basis of autoimmune diabetes in the NOD mouse.Rev Immunogenet. 2000;2(1):140-6. Rev Immunogenet. 2000. PMID: 11324686 Review.
Cited by
-
Short Duration Alagebrium Chloride Therapy Prediabetes Does Not Inhibit Progression to Autoimmune Diabetes in an Experimental Model.Metabolites. 2021 Jun 28;11(7):426. doi: 10.3390/metabo11070426. Metabolites. 2021. PMID: 34203471 Free PMC article.
-
HLA A*24:02-restricted T cell receptors cross-recognize bacterial and preproinsulin peptides in type 1 diabetes.J Clin Invest. 2024 Sep 17;134(18):e164535. doi: 10.1172/JCI164535. J Clin Invest. 2024. PMID: 39286976 Free PMC article.
-
Crinophagic granules in pancreatic β cells contribute to mouse autoimmune diabetes by diversifying pathogenic epitope repertoire.Nat Commun. 2024 Sep 27;15(1):8318. doi: 10.1038/s41467-024-52619-5. Nat Commun. 2024. PMID: 39333495 Free PMC article.
-
α-CGRP disrupts amylin fibrillization and regulates insulin secretion: implications on diabetes and migraine.Chem Sci. 2021 Mar 24;12(16):5853-5864. doi: 10.1039/d1sc01167g. Chem Sci. 2021. PMID: 34168810 Free PMC article.
-
Human insulin as both antigen and protector in type 1 diabetes.Eur J Immunol. 2024 Sep;54(9):e2350949. doi: 10.1002/eji.202350949. Epub 2024 May 22. Eur J Immunol. 2024. PMID: 38778498 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

