Distinct Evolutionary Trajectories of Neuronal and Hair Cell Nicotinic Acetylcholine Receptors
- PMID: 31821508
- PMCID: PMC7086180
- DOI: 10.1093/molbev/msz290
Distinct Evolutionary Trajectories of Neuronal and Hair Cell Nicotinic Acetylcholine Receptors
Abstract
The expansion and pruning of ion channel families has played a crucial role in the evolution of nervous systems. Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels with distinct roles in synaptic transmission at the neuromuscular junction, the central and peripheral nervous system, and the inner ear. Remarkably, the complement of nAChR subunits has been highly conserved along vertebrate phylogeny. To ask whether the different subtypes of receptors underwent different evolutionary trajectories, we performed a comprehensive analysis of vertebrate nAChRs coding sequences, mouse single-cell expression patterns, and comparative functional properties of receptors from three representative tetrapod species. We found significant differences between hair cell and neuronal receptors that were most likely shaped by the differences in coexpression patterns and coassembly rules of component subunits. Thus, neuronal nAChRs showed high degree of coding sequence conservation, coupled to greater coexpression variance and conservation of functional properties across tetrapod clades. In contrast, hair cell α9α10 nAChRs exhibited greater sequence divergence, narrow coexpression pattern, and great variability of functional properties across species. These results point to differential substrates for random change within the family of gene paralogs that relate to the segregated roles of nAChRs in synaptic transmission.
Keywords: hair cells; molecular evolution; nicotinic receptors.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures


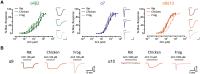

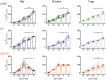


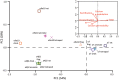
References
-
- Arendt D, Musser JM, Baker CVH, Bergman A, Cepko C, Erwin DH, Pavlicev M, Schlosser G, Widder S, Laubichler MD, et al. 2016. The origin and evolution of cell types. Nat Rev Genet. 17(12):744–757. - PubMed
-
- Cembrowski MS, Phillips MG, DiLisio SF, Shields BC, Winnubst J, Chandrashekar J, Bas E, Spruston N.. 2018. Dissociable structural and functional hippocampal outputs via distinct subiculum cell classes. Cell 173(5):1280–1292.e1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

