FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development
- PMID: 31821677
- PMCID: PMC7004512
- DOI: 10.1111/cas.14274
FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development
Abstract
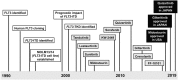
FMS-like tyrosine kinase 3 (FLT3) is a type III receptor tyrosine kinase that plays an important role in hematopoietic cell survival, proliferation and differentiation. The most clinically important point is that mutation of the FLT3 gene is the most frequent genetic alteration and a poor prognostic factor in acute myeloid leukemia (AML) patients. There are two major types of FLT3 mutations: internal tandem duplication mutations in the juxtamembrane domain (FLT3-ITD) and point mutations or deletion in the tyrosine kinase domain (FLT3-TKD). Both mutant FLT3 molecules are activated through ligand-independent dimerization and trans-phosphorylation. Mutant FLT3 induces the activation of multiple intracellular signaling pathways, mainly STAT5, MAPK and AKT signals, leading to cell proliferation and anti-apoptosis. Because high-dose chemotherapy and allogeneic hematopoietic stem cell transplantation cannot sufficiently improve the prognosis, clinical development of FLT3 kinase inhibitors expected. Although several FLT3 inhibitors have been developed, it takes more than 20 years from the first identification of FLT3 mutations until FLT3 inhibitors become clinically available for AML patients with FLT3 mutations. To date, three FLT3 inhibitors have been clinically approved as monotherapy or combination therapy with conventional chemotherapeutic agents in Japan and/or Europe and United states. However, several mechanisms of resistance to FLT3 inhibitors have already become apparent during their clinical trials. The resistance mechanisms are complex and emerging resistant clones are heterogenous. Further basic and clinical studies are required to establish the best therapeutic strategy for AML patients with FLT3 mutations.
Keywords: FMS-like tyrosine kinase; acute myeloid leukemia; inhibitor; resistance; tyrosine kinase.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Hitoshi Kiyoi received research funding from Chugai Pharmaceutical, Kyowa Hakko Kirin, Zenyaku Kogyo, FUJIFILM, Daiichi Sankyo, Astellas Pharma, Otsuka Pharmaceutical, Nippon Shinyaku, Eisai, Pfizer Japan, Takeda Pharmaceutical, Novartis Pharma KK, Sumitomo Dainippon Pharma, Sanofi KK and Celgene, consulting fees from Astellas Pharma, Amgen Astellas BioPharma KK and Daiichi Sankyo, and honoraria from Bristol‐Myers Squibb, Astellas Pharma and Novartis Pharma KK. The other authors have no conflict of interest.
Figures



References
-
- Rosnet O, Mattei MG, Marchetto S, Birnbaum D. Isolation and chromosomal localization of a novel FMS‐like tyrosine kinase gene. Genomics. 1991;9:380‐385. - PubMed
-
- Rosnet O, Schiff C, Pebusque MJ, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110‐1119. - PubMed
-
- Hannum C, Culpepper J, Campbell D, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368:643‐648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

