Adenosine Metabolism: Emerging Concepts for Cancer Therapy
- PMID: 31821783
- PMCID: PMC7224341
- DOI: 10.1016/j.ccell.2019.10.007
Adenosine Metabolism: Emerging Concepts for Cancer Therapy
Abstract
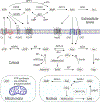
Adenosine is a key metabolic and immune-checkpoint regulator implicated in the tumor escape from the host immune system. Major gaps in knowledge that impede the development of effective adenosine-based therapeutics include: (1) lack of consideration of redundant pathways controlling ATP and adenosine levels; (2) lack of distinction between receptor-dependent and -independent effects of adenosine, and (3) focus on extracellular adenosine without consideration of intracellular metabolism and compartmentalization. In light of current clinical trials, we provide an overview of adenosine metabolism and point out the need for a more careful evaluation of the entire purinome in emerging cancer therapies.
Keywords: adenosine; adenosine kinase; adenosine receptors; biochemistry; cancer; epigenetics; immune checkpoint; intracellular; metabolism; therapy.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Allard B, Turcotte M, Spring K, Pommey S, Royal I, and Stagg J (2014). Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer 134, 1466–1473. - PubMed
-
- Antonioli L, Blandizzi C, Pacher P, and Hasko G (2013). Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 13, 842–857. - PubMed
-
- Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, Chen P, Figler H, Sullivan G, Fink S, et al. (2001). Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J 354, 123–130. - PMC - PubMed
-
- Bastid J, Regairaz A, Bonnefoy N, Dejou C, Giustiniani J, Laheurte C, Cochaud S, Laprevotte E, Funck-Brentano E, Hemon P, et al. (2015). Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res 3, 254–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

