Patient decision aids for antidepressant use in pregnancy: a pilot randomised controlled trial in the UK
- PMID: 31822489
- PMCID: PMC6995861
- DOI: 10.3399/bjgpopen19X101666
Patient decision aids for antidepressant use in pregnancy: a pilot randomised controlled trial in the UK
Abstract
Background: Decision-making regarding antidepressant use in pregnancy is challenging, given the uncertain evidence base on the benefits and risks for women and their children. Patient decision aids (PDAs) can improve shared decision-making for complex health decisions but no evidence-based PDAs exist for antidepressant use in pregnancy.
Aim: To assess the feasibility of a full-scale randomised controlled trial (RCT) to evaluate the efficacy of an electronic PDA on antidepressant use in pregnancy.
Design & setting: A UK-based pilot parallel-group RCT.
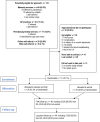
Method: The study recruited women whose clinicians recommended an antidepressant for depression in a current or planned pregnancy, and who were uncertain about antidepressant use while pregnant. Women were recruited via clinician or self-referral, and randomised to online access to the PDA or online access to standard resource list, with primary follow-up at 4 weeks and longer-term follow-up. The primary outcome was protocol feasibility (recruitment target of 50 women and follow-up rate of 80%). Outcome measures for a future full-scale RCT included the decisional conflict scale (DCS).
Results: Fifty-one women were recruited with a follow-up rate of 90.2% at 4 weeks. The PDA received good overall satisfaction ratings (mean 4.2/5). Analysis of covariance (ANCOVA) indicated a small improvement in decisional conflict at 4 weeks, accounting for baseline scores (DCS regression coefficient = -3.5, 95% confidence intervals [CI = -12.6 to 5.6]).
Conclusion: This pilot RCT for an electronic PDA on antidepressant use in pregnancy showed that the study protocol was feasible, with high rates of participant satisfaction among those randomised to the PDA.
Keywords: antidepressants; depression; patient decision aid; pilot; pregnancy; randomised controlled trial.
Copyright © 2019, The Authors.
Figures
References
-
- National Institute for Health and Care Excellence Antenatal and postnatal mental health: clinical management and service guidance. Clinical guideline [CG192] https://www.nice.org.uk/guidance/cg192. 2014 19 Nov 2019. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical