GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation
- PMID: 31822523
- PMCID: PMC7063401
- DOI: 10.1136/thoraxjnl-2019-213571
GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a rapidly progressive, fatal lung disease that affects older adults. One of the detrimental natural histories of IPF is acute exacerbation of IPF (AE-IPF), of which bacterial infection is reported to play an important role. However, the mechanism by which bacterial infection modulates the fibrotic response remains unclear.
Objectives: Altered glucose metabolism has been implicated in the pathogenesis of fibrotic lung diseases. We have previously demonstrated that glucose transporter 1 (GLUT1)-dependent glycolysis regulates fibrogenesis in a murine fibrosis model. To expand on these findings, we hypothesised that GLUT1-dependent glycolysis regulates acute exacerbation of lung fibrogenesis during bacterial infection via AIM2 inflammasome activation.
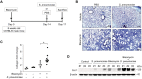
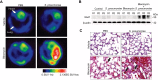
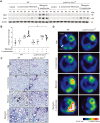
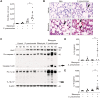
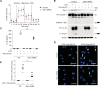
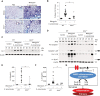
Results: In our current study, using a murine model of Streptococcus pneumoniae (S. pneumoniae) infection, we investigated the potential role of GLUT1 on mediating fibrotic responses to an acute exacerbation during bleomycin-induced fibrosis. The results of our current study illustrate that GLUT1 deficiency ameliorates S. pneumoniae-mediated exacerbation of lung fibrosis (wild type (WT)/phosphate buffered saline (PBS), n=3; WT/S. pneumoniae, n=3; WT/Bleomycin, n=5 ; WT/Bleomycin+S. pneumoniae, n=7; LysM-Cre-Glut1fl/f /PBS, n=3; LysM-Cre-Glut1fl/fl /S. pneumoniae, n=3; LysM-Cre-Glut1fl/fl /Bleomycin, n=6; LysM-Cre-Glut1fl/fl /Bleomycin+S. pneumoniae, n=9, p=0.041). Further, the AIM2 inflammasome, a multiprotein complex essential for sensing cytosolic bacterial DNA as a danger signal, is an important regulator of this GLUT1-mediated fibrosis and genetic deficiency of AIM2 reduced bleomycin-induced fibrosis after S. pneumoniae infection (WT/PBS, n=6; WT/Bleomycin+S. pneumoniae, n=15; Aim2-/-/PBS, n=6, Aim2-/-/Bleomycin+S. pneumoniae, n=11, p=0.034). GLUT1 deficiency reduced expression and function of the AIM2 inflammasome, and AIM2-deficient mice showed substantial reduction of lung fibrosis after S. pneumoniae infection.
Conclusion: Our results demonstrate that GLUT1-dependent glycolysis promotes exacerbation of lung fibrogenesis during S. pneumoniae infection via AIM2 inflammasome activation.
Keywords: idiopathic pulmonary fibrosis; interstitial fibrosis; pneumonia.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Comment in
-
Is the microbiome-induced glycolytic pathway a harbinger of acute exacerbation of idiopathic pulmonary fibrosis?Thorax. 2020 Mar;75(3):200-201. doi: 10.1136/thoraxjnl-2019-214374. Epub 2020 Jan 23. Thorax. 2020. PMID: 31974108 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
