USF1 defect drives p53 degradation during Helicobacter pylori infection and accelerates gastric carcinogenesis
- PMID: 31822580
- PMCID: PMC7456735
- DOI: 10.1136/gutjnl-2019-318640
USF1 defect drives p53 degradation during Helicobacter pylori infection and accelerates gastric carcinogenesis
Abstract
Objective: Helicobacter pylori (Hp) is a major risk factor for gastric cancer (GC). Hp promotes DNA damage and proteasomal degradation of p53, the guardian of genome stability. Hp reduces the expression of the transcription factor USF1 shown to stabilise p53 in response to genotoxic stress. We investigated whether Hp-mediated USF1 deregulation impacts p53-response and consequently genetic instability. We also explored in vivo the role of USF1 in gastric carcinogenesis.
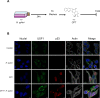
Design: Human gastric epithelial cell lines were infected with Hp7.13, exposed or not to a DNA-damaging agent camptothecin (CPT), to mimic a genetic instability context. We quantified the expression of USF1, p53 and their target genes, we determined their subcellular localisation by immunofluorescence and examined USF1/p53 interaction. Usf1-/- and INS-GAS mice were used to strengthen the findings in vivo and patient data examined for clinical relevance.
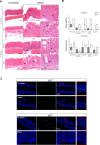
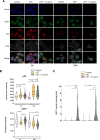
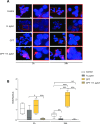
Results: In vivo we revealed the dominant role of USF1 in protecting gastric cells against Hp-induced carcinogenesis and its impact on p53 levels. In vitro, Hp delocalises USF1 into foci close to cell membranes. Hp prevents USF1/p53 nuclear built up and relocates these complexes in the cytoplasm, thereby impairing their transcriptional function. Hp also inhibits CPT-induced USF1/p53 nuclear complexes, exacerbating CPT-dependent DNA damaging effects.
Conclusion: Our data reveal that the depletion of USF1 and its de-localisation in the vicinity of cell membranes are essential events associated to the genotoxic activity of Hp infection, thus promoting gastric carcinogenesis. These findings are also of clinical relevance, supporting USF1 expression as a potential marker of GC susceptibility.
Keywords: DNA damage; gastric cancer; genetic instability; helicobacter pylori infection; oncogenes.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Comment in
-
The complicated dialogue between Helicobacter pylori and p53.Gut. 2020 Nov;69(11):2052-2053. doi: 10.1136/gutjnl-2020-320630. Epub 2020 Jan 23. Gut. 2020. PMID: 31974134 No abstract available.
References
-
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–40. - PubMed
-
- Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. . Management of epithelial precancerous conditions and lesions in the stomach (maps II): European Society of gastrointestinal endoscopy (ESGE), European Helicobacter and microbiota Study Group (EHMSG), European Society of pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019.. Endoscopy 2019;51:365–88. 10.1055/a-0859-1883 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
