Metabolic balancing by miR-276 shapes the mosquito reproductive cycle and Plasmodium falciparum development
- PMID: 31822677
- PMCID: PMC6904670
- DOI: 10.1038/s41467-019-13627-y
Metabolic balancing by miR-276 shapes the mosquito reproductive cycle and Plasmodium falciparum development
Abstract
The blood-feeding behavior of Anopheles females delivers essential nutrients for egg development and drives parasite transmission between humans. Plasmodium growth is adapted to the vector reproductive cycle, but how changes in the reproductive cycle impact parasite development remains unclear. Here, we show that the bloodmeal-induced miR-276-5p fine-tunes the expression of branched-chain amino acid transferase to terminate the reproductive cycle. Silencing of miR-276 prolongs high rates of amino acid (AA) catabolism and increases female fertility, suggesting that timely termination of AA catabolism restricts mosquito investment into reproduction. Prolongation of AA catabolism in P. falciparum-infected females also compromises the development of the transmissible sporozoite forms. Our results suggest that Plasmodium sporogony exploits the surplus mosquito resources available after reproductive investment and demonstrate the crucial role of the mosquito AA metabolism in within-vector parasite proliferation and malaria transmission.
Conflict of interest statement
The authors declare no competing interest.
Figures

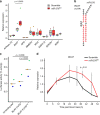
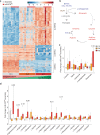


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

