The human-specific paralogs SRGAP2B and SRGAP2C differentially modulate SRGAP2A-dependent synaptic development
- PMID: 31822692
- PMCID: PMC6904453
- DOI: 10.1038/s41598-019-54887-4
The human-specific paralogs SRGAP2B and SRGAP2C differentially modulate SRGAP2A-dependent synaptic development
Abstract
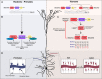
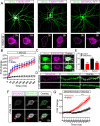
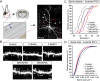
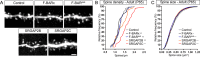
Human-specific gene duplications (HSGDs) have recently emerged as key modifiers of brain development and evolution. However, the molecular mechanisms underlying the function of HSGDs remain often poorly understood. In humans, a truncated duplication of SRGAP2A led to the emergence of two human-specific paralogs: SRGAP2B and SRGAP2C. The ancestral copy SRGAP2A limits synaptic density and promotes maturation of both excitatory (E) and inhibitory (I) synapses received by cortical pyramidal neurons (PNs). SRGAP2C binds to and inhibits all known functions of SRGAP2A leading to an increase in E and I synapse density and protracted synapse maturation, traits characterizing human cortical neurons. Here, we demonstrate how the evolutionary changes that led to the emergence of SRGAP2 HSGDs generated proteins that, in neurons, are intrinsically unstable and, upon hetero-dimerization with SRGAP2A, reduce SRGAP2A levels in a proteasome-dependent manner. Moreover, we show that, despite only a few non-synonymous mutations specifically targeting arginine residues, SRGAP2C is unique compared to SRGAP2B in its ability to induce long-lasting changes in synaptic density throughout adulthood. These mutations led to the ability of SRGAP2C to inhibit SRGAP2A function and thereby contribute to the emergence of human-specific features of synaptic development during evolution.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

