Convergent Evolution of Cysteine-Rich Keratins in Hard Skin Appendages of Terrestrial Vertebrates
- PMID: 31822906
- PMCID: PMC7086170
- DOI: 10.1093/molbev/msz279
Convergent Evolution of Cysteine-Rich Keratins in Hard Skin Appendages of Terrestrial Vertebrates
Abstract
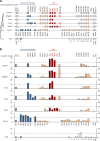
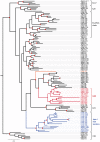
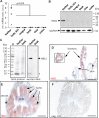
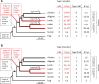
Terrestrial vertebrates have evolved hard skin appendages, such as scales, claws, feathers, and hair that play crucial roles in defense, predation, locomotion, and thermal insulation. The mechanical properties of these skin appendages are largely determined by cornified epithelial components. So-called "hair keratins," cysteine-rich intermediate filament proteins that undergo covalent cross-linking via disulfide bonds, are the crucial structural proteins of hair and claws in mammals and hair keratin orthologs are also present in lizard claws, indicating an evolutionary origin in a hairless common ancestor of amniotes. Here, we show that reptiles and birds have also other cysteine-rich keratins which lack cysteine-rich orthologs in mammals. In addition to hard acidic (type I) sauropsid-specific (HAS) keratins, we identified hard basic (type II) sauropsid-specific (HBS) keratins which are conserved in lepidosaurs, turtles, crocodilians, and birds. Immunohistochemical analysis with a newly made antibody revealed expression of chicken HBS1 keratin in the cornifying epithelial cells of feathers. Molecular phylogenetics suggested that the high cysteine contents of HAS and HBS keratins evolved independently from the cysteine-rich sequences of hair keratin orthologs, thus representing products of convergent evolution. In conclusion, we propose an evolutionary model in which HAS and HBS keratins evolved as structural proteins in epithelial cornification of reptiles and at least one HBS keratin was co-opted as a component of feathers after the evolutionary divergence of birds from reptiles. Thus, cytoskeletal proteins of hair and feathers are products of convergent evolution and evolutionary co-option to similar biomechanical functions in clade-specific hard skin appendages.
Keywords: co-option; convergent evolution; feathers; gene family; keratin.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures


References
-
- Alibardi L. 2016. The process of cornification evolved from the initial keratinization in the epidermis and epidermal derivatives of vertebrates: a new synthesis and the case of sauropsids. Int Rev Cell Mol Biol. 327:263–319. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ.. 1990. Basic local alignment search tool. J Mol Biol. 215(3):403–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

