Silencing of IL13RA2 promotes partial epithelial-mesenchymal transition in hepatocellular carcinoma via ERK signaling pathway activation
- PMID: 31823484
- PMCID: PMC6996351
- DOI: 10.1002/2211-5463.12774
Silencing of IL13RA2 promotes partial epithelial-mesenchymal transition in hepatocellular carcinoma via ERK signaling pathway activation
Abstract
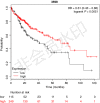
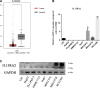
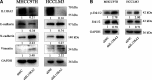
Lack of insight into the mechanisms underlying hepatocellular carcinoma (HCC) metastasis has hindered the development of curative treatments. Overexpression of interleukin-13 receptor alpha 2 (IL13RA2) has been reported to contribute to invasion and metastasis in several tumors. However, the role of IL13RA2 in HCC remains to be characterized. In this study, we identified that low expression of IL13RA2 is associated with poor survival of patients with HCC, and demonstrated that IL13RA2 knockdown endows HCC cells with invasive potential. Mechanistically, silencing of IL13RA2 promotes partial epithelial-mesenchymal transition via increasing extracellular signal-regulated kinase phosphorylation in HCC. Collectively, our results suggest that IL13RA2 may have potential as a prognostic biomarker for HCC.
Keywords: ERK signaling; epithelial-mesenchymal transition; hepatocellular carcinoma; interleukin-13 receptor alpha 2.
© 2019 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424. - PubMed
-
- Llovet JM, Bru C and Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19, 329–338. - PubMed
-
- Niu L, Liu L, Yang S, Ren J, Lai P and Chen GG (2017) New insights into sorafenib resistance in hepatocellular carcinoma: responsible mechanisms and promising strategies. Biochim Biophys Acta Rev Cancer 1868, 564–570. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359, 378–390. - PubMed
-
- Andrews AL, Holloway JW, Puddicombe SM, Holgate ST and Davies DE (2002) Kinetic analysis of the interleukin‐13 receptor complex. J Biol Chem 277, 46073–46078. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

