A Survival Guide for the "Electro-curious"
- PMID: 31823612
- PMCID: PMC6996934
- DOI: 10.1021/acs.accounts.9b00539
A Survival Guide for the "Electro-curious"
Abstract
The appeal and promise of synthetic organic electrochemistry have been appreciated over the past century. In terms of redox chemistry, which is frequently encountered when forging new bonds, it is difficult to conceive of a more economical way to add or remove electrons than electrochemistry. Indeed, many of the largest industrial synthetic chemical processes are achieved in a practical way using electrons as a reagent. Why then, after so many years of the documented benefits of electrochemistry, is it not more widely embraced by mainstream practitioners? Erroneous perceptions that electrochemistry is a "black box" combined with a lack of intuitive and inexpensive standardized equipment likely contributed to this stagnation in interest within the synthetic organic community. This barrier to entry is magnified by the fact that many redox processes can already be accomplished using simple chemical reagents even if they are less atom-economic. Time has proven that sustainability and economics are not strong enough driving forces for the adoption of electrochemical techniques within the broader community. Indeed, like many synthetic organic chemists that have dabbled in this age-old technique, our first foray into this area was not by choice but rather through sheer necessity. The unique reactivity benefits of this old redox-modulating technique must therefore be highlighted and leveraged in order to draw organic chemists into the field. Enabling new bonds to be forged with higher levels of chemo- and regioselectivity will likely accomplish this goal. In doing so, it is envisioned that widespread adoption of electrochemistry will go beyond supplanting unsustainable reagents in mundane redox reactions to the development of exciting reactivity paradigms that enable heretofore unimagined retrosynthetic pathways. Whereas the rigorous physical organic chemical principles of electroorganic synthesis have been reviewed elsewhere, it is often the case that such summaries leave out the pragmatic aspects of designing, optimizing, and scaling up preparative electrochemical reactions. Taken together, the task of setting up an electrochemical reaction, much less inventing a new one, can be vexing for even seasoned organic chemists. This Account therefore features a unique format that focuses on addressing this exact issue within the context of our own studies. The graphically rich presentation style pinpoints basic concepts, typical challenges, and key insights for those "electro-curious" chemists who seek to rapidly explore the power of electrochemistry in their research.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
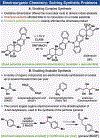





References
-
- Moeller KD Synthetic Applications of Anodic Electrochemistry. Tetrahedron 2000, 56, 9527–9554.
-
- Sperry JB; Wright DL The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules. Chem. Soc. Rev 2006, 35, 605–621. - PubMed
-
- Utley J Trends in organic electrosynthesis. Chem. Soc. Rev 1997, 26, 157–167.
-
- Kärkäs MD Electrochemical strategies for C–H functionalization and C–N bond formation. Chem. Soc. Rev 2018, 47, 5786–5865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

