The multi-faceted functioning portrait of LRF/ZBTB7A
- PMID: 31823818
- PMCID: PMC6905007
- DOI: 10.1186/s40246-019-0252-0
The multi-faceted functioning portrait of LRF/ZBTB7A
Abstract
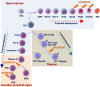
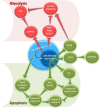
Transcription factors (TFs) consisting of zinc fingers combined with BTB (for broad-complex, tram-track, and bric-a-brac) domain (ZBTB) are a highly conserved protein family that comprises a multifunctional and heterogeneous group of TFs, mainly modulating cell developmental events and cell fate. LRF/ZBTB7A, in particular, is reported to be implicated in a wide variety of physiological and cancer-related cell events. These physiological processes include regulation of erythrocyte maturation, B/T cell differentiation, adipogenesis, and thymic insulin expression affecting consequently insulin self-tolerance. In cancer, LRF/ZBTB7A has been reported to act either as oncogenic or as oncosuppressive factor by affecting specific cell processes (proliferation, apoptosis, invasion, migration, metastasis, etc) in opposed ways, depending on cancer type and molecular interactions. The molecular mechanisms via which LRF/ZBTB7A is known to exert either physiological or cancer-related cellular effects include chromatin organization and remodeling, regulation of the Notch signaling axis, cellular response to DNA damage stimulus, epigenetic-dependent regulation of transcription, regulation of the expression and activity of NF-κB and p53, and regulation of aerobic glycolysis and oxidative phosphorylation (Warburg effect). It is a pleiotropic TF, and thus, alterations to its expression status become detrimental for cell survival. This review summarizes its implication in different cellular activities and the commonly invoked molecular mechanisms triggered by LRF/ZBTB7A's orchestrated action.
Keywords: Adipogenesis; Apoptosis; Glycolysis; Hematopoietic stem cell differentiation; Oncogene; Thymic insulin expression; Tumor-suppressor gene; Zinc finger and BTB domain transcription factor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Ramos Pittol José Miguel, Oruba Agata, Mittler Gerhard, Saccani Simona, van Essen Dominic. Zbtb7a is a transducer for the control of promoter accessibility by NF-kappa B and multiple other transcription factors. PLOS Biology. 2018;16(5):e2004526. doi: 10.1371/journal.pbio.2004526. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

