Adult neurogenesis from reprogrammed astrocytes
- PMID: 31823866
- PMCID: PMC7034263
- DOI: 10.4103/1673-5374.270292
Adult neurogenesis from reprogrammed astrocytes
Abstract
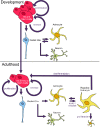
The details of adult neurogenesis, including environmental triggers, region specificity, and species homology remain an area of intense investigation. Slowing or halting age-related cognitive dysfunction, or restoring neurons lost to disease or injury represent just a fraction of potential therapeutic applications. New neurons can derive from stem cells, pluripotent neural progenitor cells, or non-neuronal glial cells, such as astrocytes. Astrocytes must be epigenetically "reprogrammed" to become neurons, which can occur both naturally in vivo, and via artificial exogenous treatments. While neural progenitor cells are localized to a few neurogenic zones in the adult brain, astrocytes populate almost every brain structure. In this review, we will summarize recent research into neurogenesis that arises from conversion of post-mitotic astrocytes, detail the genetic and epigenetic pathways that regulate this process, and discuss the possible clinical relevance in supplementing stem-cell neurogenic therapies.
Keywords: astrocyte; brain; dedifferentiation; development; disease; glia; injury; neurogenesis.
Conflict of interest statement
None
Figures

References
-
- Aravantinou-Fatorou K, Ortega F, Chroni-Tzartou D, Antoniou N, Poulopoulou C, Politis PK, Berninger B, Matsas R, Thomaidou D. CEND1 and NEUROGENIN2 reprogram mouse astrocytes and embryonic fibroblasts to induced neural precursors and differentiated neurons. Stem Cell Rep. 2015;5:405–418. - PMC - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and Ink4a/Arf: Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

