RU486 Metabolite Inhibits CCN1/Cyr61 Secretion by MDA-MB-231-Endothelial Adhesion
- PMID: 31824306
- PMCID: PMC6880622
- DOI: 10.3389/fphar.2019.01296
RU486 Metabolite Inhibits CCN1/Cyr61 Secretion by MDA-MB-231-Endothelial Adhesion
Abstract
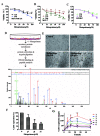
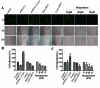
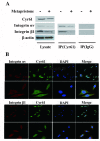
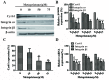
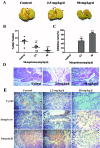
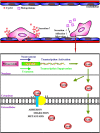
Successful adhesion of circulating tumor cells (CTCs) to microvascular endothelium of distant metastatic tissue is the key starting step of metastatic cascade that could be effectively chemoprevented as we demonstrated previously. Here, we hypothesize that the hetero-adhesion may produce secretory biomarkers that may be important for both premetastatic diagnosis and chemoprevention. We show that co-incubation of triple-negative breast cancer (TNBC) cell line MDA-MB-231 with human pulmonary microvascular endothelial monolayers (HPMEC) secretes Cyr61 (CCN1), primarily from MDA-MB-231. However, addition of metapristone (RU486 metabolite) to the co-incubation system inhibits Cyr61 secretion probably via the Cyr61/integrin αvβ1 signaling pathway without significant cytotoxicity on both MDA-MB-231 and HPMEC. Transfection of MDA-MB-231 with Cyr61-related recombinant plasmid or siRNA enhances or reduces Cyr61 expression, accordingly. The transfection significantly changes hetero-adhesion and migration of MDA-MB-231, and the changed bioactivities by overexpressed CYR61 could be antagonized by metapristone in vitro. Moreover, the circulating MDA-MB-231 develops lung metastasis in mice, which could be effectively prevented by oral metapristone without significant toxicity. The present study, for the first time, demonstrates that co-incubation of MDA-MB-231 with HPMEC secrets CYR61 probably via the CYR61/integrin αvβ1 signaling pathway to promote adhesion-invasion of TNBC (early metastatic step). Metapristone, by interfering the adhesion-invasion process, prevents metastasis from happening.
Keywords: CYR61; MDA-MB-231/HPMEC co-culture; integrin αvβ1; metapristone; metastasis chemoprevention.
Copyright © 2019 Yu, Yan, Wu, He, Liu, Liu, Yang, Ma, Lu and Jia.
Figures
Similar articles
-
Pharmacoproteomic analysis reveals that metapristone (RU486 metabolite) intervenes E-cadherin and vimentin to realize cancer metastasis chemoprevention.Sci Rep. 2016 Mar 2;6:22388. doi: 10.1038/srep22388. Sci Rep. 2016. PMID: 26932781 Free PMC article.
-
Abortifacient metapristone (RU486 derivative) interrupts CXCL12/CXCR4 axis for ovarian metastatic chemoprevention.Mol Carcinog. 2017 Aug;56(8):1896-1908. doi: 10.1002/mc.22645. Epub 2017 Mar 30. Mol Carcinog. 2017. PMID: 28277622
-
The matricellular protein CYR61 promotes breast cancer lung metastasis by facilitating tumor cell extravasation and suppressing anoikis.Oncotarget. 2017 Feb 7;8(6):9200-9215. doi: 10.18632/oncotarget.13677. Oncotarget. 2017. PMID: 27911269 Free PMC article.
-
Cyr61 as mediator of Src signaling in triple negative breast cancer cells.Oncotarget. 2015 May 30;6(15):13520-38. doi: 10.18632/oncotarget.3760. Oncotarget. 2015. PMID: 25980494 Free PMC article.
-
In vitro and in vivo efficacy and safety evaluation of metapristone and mifepristone as cancer metastatic chemopreventive agents.Biomed Pharmacother. 2016 Mar;78:291-300. doi: 10.1016/j.biopha.2016.01.017. Epub 2016 Feb 4. Biomed Pharmacother. 2016. PMID: 26898454
Cited by
-
The Potential of Hormonal Therapies for Treatment of Triple-Negative Breast Cancer.Cancers (Basel). 2023 Sep 24;15(19):4702. doi: 10.3390/cancers15194702. Cancers (Basel). 2023. PMID: 37835396 Free PMC article. Review.
-
The Crosstalk Analysis between mPSCs and Panc1 Cells Identifies CCN1 as a Positive Regulator of Gemcitabine Sensitivity in Pancreatic Cancer Cells.Int J Mol Sci. 2024 Aug 29;25(17):9369. doi: 10.3390/ijms25179369. Int J Mol Sci. 2024. PMID: 39273316 Free PMC article.
-
Potential Role of CCN Proteins in Breast Cancer: Therapeutic Advances and Perspectives.Curr Oncol. 2021 Nov 26;28(6):4972-4985. doi: 10.3390/curroncol28060417. Curr Oncol. 2021. PMID: 34940056 Free PMC article. Review.
-
Metapristone (RU486-derivative) inhibits endometrial cancer cell progress through regulating miR-492/Klf5/Nrf1 axis.Cancer Cell Int. 2021 Jan 7;21(1):29. doi: 10.1186/s12935-020-01682-1. Cancer Cell Int. 2021. PMID: 33413440 Free PMC article.
-
Micro RNA-175 Targets Claudin-1 to Inhibit Madin-Darby Canine Kidney Cell Adhesion.Genes (Basel). 2024 Oct 16;15(10):1333. doi: 10.3390/genes15101333. Genes (Basel). 2024. PMID: 39457456 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

