Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit
- PMID: 31824934
- PMCID: PMC6882937
- DOI: 10.3389/fbioe.2019.00337
Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit
Abstract
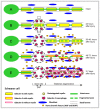
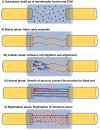
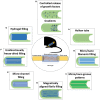
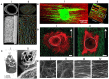
Peripheral nerve repair and regeneration remains among the greatest challenges in tissue engineering and regenerative medicine. Even though peripheral nerve injuries (PNIs) are capable of some degree of regeneration, frail recovery is seen even when the best microsurgical technique is applied. PNIs are known to be very incapacitating for the patient, due to the deprivation of motor and sensory abilities. Since there is no optimal solution for tackling this problem up to this day, the evolution in the field is constant, with innovative designs of advanced nerve guidance conduits (NGCs) being reported every day. As a basic concept, a NGC should act as a physical barrier from the external environment, concomitantly acting as physical guidance for the regenerative axons across the gap lesion. NGCs should also be able to retain the naturally released nerve growth factors secreted by the damaged nerve stumps, as well as reducing the invasion of scar tissue-forming fibroblasts to the injury site. Based on the neurobiological knowledge related to the events that succeed after a nerve injury, neuronal subsistence is subjected to the existence of an ideal environment of growth factors, hormones, cytokines, and extracellular matrix (ECM) factors. Therefore, it is known that multifunctional NGCs fabricated through combinatorial approaches are needed to improve the functional and clinical outcomes after PNIs. The present work overviews the current reports dealing with the several features that can be used to improve peripheral nerve regeneration (PNR), ranging from the simple use of hollow NGCs to tissue engineered intraluminal fillers, or to even more advanced strategies, comprising the molecular and gene therapies as well as cell-based therapies.
Keywords: biomaterials; luminal fillers; nerve guidance conduit; peripheral nerve; tissue engineering.
Copyright © 2019 Carvalho, Oliveira and Reis.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

