Mitochondria in Embryogenesis: An Organellogenesis Perspective
- PMID: 31824944
- PMCID: PMC6883342
- DOI: 10.3389/fcell.2019.00282
Mitochondria in Embryogenesis: An Organellogenesis Perspective
Abstract
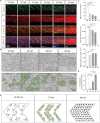
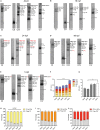
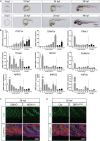
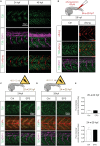
Organogenesis is well characterized in vertebrates. However, the anatomical and functional development of intracellular compartments during this phase of development remains unknown. Taking an organellogenesis point of view, we characterize the spatiotemporal adaptations of the mitochondrial network during zebrafish embryogenesis. Using state of the art microscopy approaches, we find that mitochondrial network follows three distinct distribution patterns during embryonic development. Despite of this constant morphological change of the mitochondrial network, electron transport chain supercomplexes occur at early stages of embryonic development and conserve a stable organization throughout development. The remodeling of the mitochondrial network and the conservation of its structural components go hand-in-hand with somite maturation; for example, genetic disruption of myoblast fusion impairs mitochondrial network maturation. Reciprocally, mitochondria quality represents a key factor to determine embryonic progression. Alteration of mitochondrial polarization and electron transport chain halts embryonic development in a reversible manner suggesting developmental checkpoints that depend on mitochondrial integrity. Our findings establish the subtle dialogue and co-dependence between organogenesis and mitochondria in early vertebrate development. They also suggest the importance of adopting subcellular perspectives to understand organelle-organ communications during embryogenesis.
Keywords: electron transport chain supercomplexes; mitochondria fission; morphogenes; myomaker; somite; sonic hedgehog; zebrafish.
Copyright © 2019 Arribat, Grepper, Lagarrigue, Richard, Gachet, Gut and Amati.
Figures



References
-
- Alam M. M., Sohoni S., Kalainayakan S. P., Garrossian M., Zhang L. (2016). Cyclopamine tartrate, an inhibitor of Hedgehog signaling, strongly interferes with mitochondrial function and suppresses aerobic respiration in lung cancer cells. BMC Cancer 16:150. 10.1186/s12885-016-2200-x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

