Spatiotemporal dynamics of pial collateral blood flow following permanent middle cerebral artery occlusion in a rat model of sensory-based protection: a Doppler optical coherence tomography study
- PMID: 31824979
- PMCID: PMC6903432
- DOI: 10.1117/1.NPh.6.4.045012
Spatiotemporal dynamics of pial collateral blood flow following permanent middle cerebral artery occlusion in a rat model of sensory-based protection: a Doppler optical coherence tomography study
Abstract
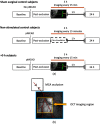
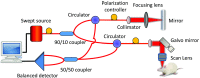
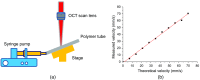
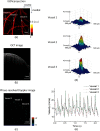
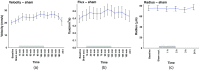
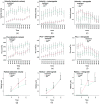
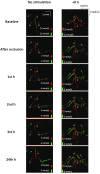
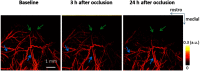
There is a growing recognition regarding the importance of pial collateral flow in the protection from impending ischemic stroke both in preclinical and clinical studies. Collateral flow is also a major player in sensory stimulation-based protection from impending ischemic stroke. Doppler optical coherence tomography has been employed to image spatiotemporal patterns of collateral flow within the dorsal branches of the middle cerebral artery (MCA) as it provides a powerful tool for quantitative in vivo flow parameters imaging (velocity, flux, direction of flow, and radius of imaged branches). It was employed prior to and following dorsal permanent MCA occlusion (pMCAo) in rat models of treatment by protective sensory stimulation, untreated controls, or sham surgery controls. Unexpectedly, following pMCAo in the majority of subjects, some MCA branches continued to show anterograde blood flow patterns over time despite severing of the MCA. Further, in the presence of protective sensory stimulation, the anterograde velocity and flux were stronger and lasted longer than in retrograde flow branches, even within different branches of single subjects, but stimulated retrograde branches showed stronger flow parameters at 24 h. Our study suggests that the spatiotemporal patterns of collateral-based dorsal MCA flow are dynamic and provide a detailed description on the differential effects of protective sensory stimulation.
Keywords: Doppler optical coherence tomography; cortex; leptomeningeal anastomoses; leptomeningeal collaterals; neurovascular.
© The Authors. Published by SPIE under a Creative Commons Attribution 4.0 Unported License. Distribution or reproduction of this work in whole or in part requires full attribution of the original publication, including its DOI.
Figures