High CD206 levels in Hodgkin lymphoma-educated macrophages are linked to matrix-remodeling and lymphoma dissemination
- PMID: 31825135
- PMCID: PMC7053241
- DOI: 10.1002/1878-0261.12616
High CD206 levels in Hodgkin lymphoma-educated macrophages are linked to matrix-remodeling and lymphoma dissemination
Abstract
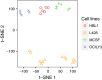
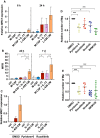
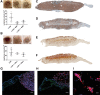
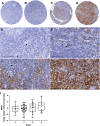
Macrophages (Mφ) are abundantly present in the tumor microenvironment and may predict outcome in solid tumors and defined lymphoma subtypes. Mφ heterogeneity, the mechanisms of their recruitment, and their differentiation into lymphoma-promoting, alternatively activated M2-like phenotypes are still not fully understood. Therefore, further functional studies are required to understand biological mechanisms associated with human tumor-associated Mφ (TAM). Here, we show that the global mRNA expression and protein abundance of human Mφ differentiated in Hodgkin lymphoma (HL)-conditioned medium (CM) differ from those of Mφ educated by conditioned media from diffuse large B-cell lymphoma (DLBCL) cells or, classically, by macrophage colony-stimulating factor (M-CSF). Conditioned media from HL cells support TAM differentiation through upregulation of surface antigens such as CD40, CD163, CD206, and PD-L1. In particular, RNA and cell surface protein expression of mannose receptor 1 (MRC1)/CD206 significantly exceed the levels induced by classical M-CSF stimulation in M2-like Mφ; this is regulated by interleukin 13 to a large extent. Functionally, high CD206 enhances mannose-dependent endocytosis and uptake of type I collagen. Together with high matrix metalloprotease9 secretion, HL-TAMs appear to be active modulators of the tumor matrix. Preclinical in ovo models show that co-cultures of HL cells with monocytes or Mφ support dissemination of lymphoma cells via lymphatic vessels, while tumor size and vessel destruction are decreased in comparison with lymphoma-only tumors. Immunohistology of human HL tissues reveals a fraction of cases feature large numbers of CD206-positive cells, with high MRC1 expression being characteristic of HL-stage IV. In summary, the lymphoma-TAM interaction contributes to matrix-remodeling and lymphoma cell dissemination.
Keywords: CD206; lymphoma; macrophages; tumor microenvironment.
© 2019 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Aldinucci D, Gloghini A, Pinto A, De Filippi R and Carbone A (2010) The classical Hodgkin’s lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol 221, 248–263. - PubMed
-
- Allen JE and Rückerl D (2017) The silent undertakers: macrophages programmed for efferocytosis. Immunity 47, 810–812. - PubMed
-
- Azad AK and Schlesinger LS (2015) Mannose receptor (CD206)‐mediated imaging in sentinel lymph node localization. Clin Transl Imaging 3, 237–245.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

