Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism
- PMID: 31825657
- PMCID: PMC7099524
- DOI: 10.1152/ajpcell.00540.2019
Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism
Abstract
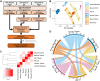
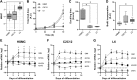
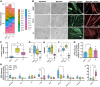
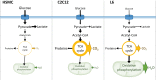
Rat L6, mouse C2C12, and primary human skeletal muscle cells (HSMCs) are commonly used to study biological processes in skeletal muscle, and experimental data on these models are abundant. However, consistently matched experimental data are scarce, and comparisons between the different cell types and adult tissue are problematic. We hypothesized that metabolic differences between these cellular models may be reflected at the mRNA level. Publicly available data sets were used to profile mRNA levels in myotubes and skeletal muscle tissues. L6, C2C12, and HSMC myotubes were assessed for proliferation, glucose uptake, glycogen synthesis, mitochondrial activity, and substrate oxidation, as well as the response to in vitro contraction. Transcriptomic profiling revealed that mRNA of genes coding for actin and myosin was enriched in C2C12, whereas L6 myotubes had the highest levels of genes encoding glucose transporters and the five complexes of the mitochondrial electron transport chain. Consistently, insulin-stimulated glucose uptake and oxidative capacity were greatest in L6 myotubes. Insulin-induced glycogen synthesis was highest in HSMCs, but C2C12 myotubes had higher baseline glucose oxidation. All models responded to electrical pulse stimulation-induced glucose uptake and gene expression but in a slightly different manner. Our analysis reveals a great degree of heterogeneity in the transcriptomic and metabolic profiles of L6, C2C12, or primary human myotubes. Based on these distinct signatures, we provide recommendations for the appropriate use of these models depending on scientific hypotheses and biological relevance.
Keywords: C2C12; metabolism; skeletal muscle; transcriptomics.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Berg JM, Tymoczko JL, Stryer L. Amino acids are made from intermediates of the citric acid cycle and other major pathways. In: Biochemistry (5th ed.). New York: WH Freeman, 2002. https://www.ncbi.nlm.nih.gov/books/NBK22459/.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

