Outcome in Children With Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children's Oncology Group Trial AALL0331
- PMID: 31825704
- PMCID: PMC7030893
- DOI: 10.1200/JCO.19.01086
Outcome in Children With Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children's Oncology Group Trial AALL0331
Abstract
Purpose: Children's Oncology Group (COG) AALL0331 tested whether intensified postinduction therapy that improves survival in children with high-risk B-cell acute lymphoblastic leukemia (ALL) would also improve outcomes for those with standard-risk (SR) ALL.
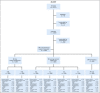
Patients and methods: AALL0331 enrolled 5,377 patients between 2005 and 2010. All patients received a 3-drug induction with dexamethasone, vincristine, and pegaspargase (PEG) and were then classified as SR low, SR average, or SR high. Patients with SR-average disease were randomly assigned to receive either standard 4-week consolidation (SC) or 8-week intensified augmented Berlin-Frankfurt-Münster (BFM) consolidation (IC). Those with SR-high disease were nonrandomly assigned to the full COG-augmented BFM regimen, including 2 interim maintenance and delayed intensification phases.
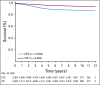
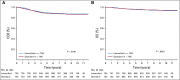
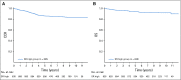
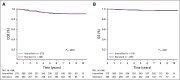
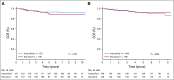
Results: The 6-year event-free survival (EFS) rate for all patients enrolled in AALL0331 was 88.96% ± 0.46%, and overall survival (OS) was 95.54% ± 0.31%. For patients with SR-average disease, the 6-year continuous complete remission (CCR) and OS rates for SC versus IC were 87.8% ± 1.3% versus 89.1% ± 1.2% (P = .52) and 95.8% ± 0.8% versus 95.2% ± 0.8% (P = 1.0), respectively. Those with SR-average disease with end-induction minimal residual disease (MRD) of 0.01% to < 0.1% had an inferior outcome compared with those with lower MRD and no improvement with IC (6-year CCR: SC, 77.5% ± 4.8%; IC, 77.1% ± 4.8%; P = .71). At 6 years, the CCR and OS rates among 635 nonrandomly treated patients with SR-high disease were 85.55% ± 1.49% and 92.97% ± 1.08%, respectively.
Conclusion: The 6-year OS rate for > 5,000 children with SR ALL enrolled in AALL0331 exceeded 95%. The addition of IC to treatment for patients with SR-average disease did not improve CCR or OS, even in patients with higher MRD, in whom it might have been predicted to provide more value. The EFS and OS rates are excellent for this group of patients with SR ALL, with particularly good outcomes for those with SR-high disease.
Figures
References
-
- Gaynon PS, Bleyer WA, Steinherz PG, et al. Modified BFM therapy for children with previously untreated acute lymphoblastic leukemia and unfavorable prognostic features: Report of Children’s Cancer Study Group study CCG-193P. Am J Pediatr Hematol Oncol. 1988;10:42–50. - PubMed
-
- Eden OB, Harrison G, Richards S, et al. Long-term follow-up of the United Kingdom Medical Research Council protocols for childhood acute lymphoblastic leukaemia, 1980-1997. Leukemia. 2000;14:2307–2320. - PubMed
-
- Chessells JM, Bailey C, Richards SM. Intensification of treatment and survival in all children with lymphoblastic leukaemia: Results of UK Medical Research Council trial UKALL X. Lancet. 1995;345:143–148. - PubMed
-
- Schrappe M, Reiter A, Zimmermann M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995: Berlin-Frankfurt-Münster. Leukemia. 2000;14:2205–2222. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

