Deep Phenotyping of PDE6C-Associated Achromatopsia
- PMID: 31826238
- PMCID: PMC6905659
- DOI: 10.1167/iovs.19-27761
Deep Phenotyping of PDE6C-Associated Achromatopsia
Abstract
Purpose: To perform deep phenotyping of subjects with PDE6C achromatopsia and examine disease natural history.
Methods: Eight subjects with disease-causing variants in PDE6C were assessed in detail, including clinical phenotype, best-corrected visual acuity, fundus autofluorescence, and optical coherence tomography. Six subjects also had confocal and nonconfocal adaptive optics scanning light ophthalmoscopy, axial length, international standard pattern and full-field electroretinography (ERG), short-wavelength flash (S-cone) ERGs, and color vision testing.
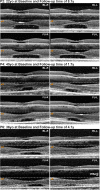
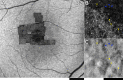
Results: All subjects presented with early-onset nystagmus, decreased best-corrected visual acuity, light sensitivity, and severe color vision loss, and five of them had high myopia. We identified three novel disease-causing variants and provide phenotype data associated with nine variants for the first time. No subjects had foveal hypoplasia or residual ellipsoid zone (EZ) at the foveal center; one had an absent EZ, three had a hyporeflective zone, and four had outer retinal atrophy. The mean width of the central EZ lesion on optical coherence tomography at baseline was 1923 μm. The mean annual increase in EZ lesion size was 48.3 μm. Fundus autofluorescence revealed a central hypoautofluorescence with a surrounding ring of increased signal (n = 5). The mean hypoautofluorescent area at baseline was 3.33 mm2 and increased in size by a mean of 0.13 mm2/year. Nonconfocal adaptive optics scanning light ophthalmoscopy revealed residual foveal cones in only one of two cases. Full-field ERGs were consistent with severe generalized cone system dysfunction but with relative preservation of S-cone sensitivity.
Conclusions: PDE6C retinopathy is a severe cone dysfunction syndrome often presenting as typical achromatopsia but without foveal hypoplasia. Myopia and slowly progressive maculopathy are common features. There are few (if any) residual foveal cones for intervention in older adults.
Figures





References
-
- Hirji N, Aboshiha J, Georgiou M, et al. Achromatopsia: clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet. 2018;39:1–9. - PubMed
-
- Wissinger B, Jagle H, Kohl S, et al. Human rod monochromacy: linkage analysis and mapping of a cone photoreceptor expressed candidate gene on chromosome 2q11. Genomics. 1998;51:325–331. - PubMed
-
- Mayer AK, Van Cauwenbergh C, Rother C, et al. CNGB3 mutation spectrum including copy number variations in 552 achromatopsia patients. Hum Mutat. 2017;38:1579–1591. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

