Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients
- PMID: 31826336
- PMCID: PMC7306117
- DOI: 10.1056/NEJMoa1911124
Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients
Abstract
Background: Vitamin D deficiency is a common, potentially reversible contributor to morbidity and mortality among critically ill patients. The potential benefits of vitamin D supplementation in acute critical illness require further study.
Methods: We conducted a randomized, double-blind, placebo-controlled, phase 3 trial of early vitamin D3 supplementation in critically ill, vitamin D-deficient patients who were at high risk for death. Randomization occurred within 12 hours after the decision to admit the patient to an intensive care unit. Eligible patients received a single enteral dose of 540,000 IU of vitamin D3 or matched placebo. The primary end point was 90-day all-cause, all-location mortality.
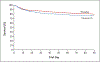
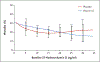
Results: A total of 1360 patients were found to be vitamin D-deficient during point-of-care screening and underwent randomization. Of these patients, 1078 had baseline vitamin D deficiency (25-hydroxyvitamin D level, <20 ng per milliliter [50 nmol per liter]) confirmed by subsequent testing and were included in the primary analysis population. The mean day 3 level of 25-hydroxyvitamin D was 46.9±23.2 ng per milliliter (117±58 nmol per liter) in the vitamin D group and 11.4±5.6 ng per milliliter (28±14 nmol per liter) in the placebo group (difference, 35.5 ng per milliliter; 95% confidence interval [CI], 31.5 to 39.6). The 90-day mortality was 23.5% in the vitamin D group (125 of 531 patients) and 20.6% in the placebo group (109 of 528 patients) (difference, 2.9 percentage points; 95% CI, -2.1 to 7.9; P = 0.26). There were no clinically important differences between the groups with respect to secondary clinical, physiological, or safety end points. The severity of vitamin D deficiency at baseline did not affect the association between the treatment assignment and mortality.
Conclusions: Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other, nonfatal outcomes among critically ill, vitamin D-deficient patients. (Funded by the National Heart, Lung, and Blood Institute; VIOLET ClinicalTrials.gov number, NCT03096314.).
Copyright © 2019 Massachusetts Medical Society.
Figures
Comment in
-
High-Dose Vitamin D3 for Critically Ill Vitamin D-Deficient Patients.N Engl J Med. 2020 Apr 23;382(17):1669. doi: 10.1056/NEJMc2000993. N Engl J Med. 2020. PMID: 32320582 No abstract available.
-
High-Dose Vitamin D3 for Critically Ill Vitamin D-Deficient Patients.N Engl J Med. 2020 Apr 23;382(17):1669-1670. doi: 10.1056/NEJMc2000993. N Engl J Med. 2020. PMID: 32320583 No abstract available.
-
High-Dose Vitamin D3 for Critically Ill Vitamin D-Deficient Patients.N Engl J Med. 2020 Apr 23;382(17):1670. doi: 10.1056/NEJMc2000993. N Engl J Med. 2020. PMID: 32320584 No abstract available.
References
-
- Takano Y, Mitsuhashi H, Ueno K. 1α,25-Dihydroxyvitamin D3 inhibits neutrophil recruitment in hamster model of acute lung injury. Steroids 2011;76:1305–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL123018/HL/NHLBI NIH HHS/United States
- U01 HL123031/HL/NHLBI NIH HHS/United States
- U01 HL123020/HL/NHLBI NIH HHS/United States
- U01 HL122989/HL/NHLBI NIH HHS/United States
- U01 HL123004/HL/NHLBI NIH HHS/United States
- U01 HL123008/HL/NHLBI NIH HHS/United States
- U01 HL123023/HL/NHLBI NIH HHS/United States
- U01 HL122998/HL/NHLBI NIH HHS/United States
- U01HL123008, U01HL123031, U01HL123004, U01HL123027/US/United States/United States
- U01 HL123009/HL/NHLBI NIH HHS/United States
- U01HL123009, U01HL122998, U01HL123018, U01HL123023/US/United States/United States
- U01HL123010, U01HL123033, U01HL122989, U01HL123022/US/United States/United States
- U01 HL123022/HL/NHLBI NIH HHS/United States
- U01 HL123027/HL/NHLBI NIH HHS/United States
- U01HL123020/US/United States/United States
- U01 HL123033/HL/NHLBI NIH HHS/United States
- U01 HL123010/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
