Anticancer drug prices and clinical outcomes: a cross-sectional study in Italy
- PMID: 31826897
- PMCID: PMC6924817
- DOI: 10.1136/bmjopen-2019-033728
Anticancer drug prices and clinical outcomes: a cross-sectional study in Italy
Abstract
Objective: To investigate whether the prices of new anticancer drugs correlated with their relative benefit despite negotiation.
Design: Retrospective cross-sectional study correlating new anticancer drugs prices with clinical outcomes.
Setting: We did a retrospective cross-sectional study including all new anticancer drugs approved by the European Medicines Agency (EMA) (2010-2016) and reimbursed in Italy.
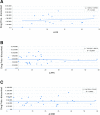
Main outcomes and measures: Information on clinical outcomes-in terms of median overall survival (OS), median progression-free survival (PFS) and objective response rate (ORR)-was extracted from pivotal trials as reported in the European Public Assessment Reports available on the EMA website. Cost of a full course treatment was estimated on negotiated official and discounted prices. Regression coefficients β, their levels of significance p and the coefficients of determination R2 were estimated adjusting by tumour type.
Results: Overall, 30 new anticancer drugs (with 35 indications) were available for analysis. Where data on OS were available, we observed no correlation between the improvement in median OS (in weeks) and negotiated price (R2=0.067, n=16 drugs for 17 indications). When the clinical outcomes were expressed as improvements in the median PFS or ORR, 25 drugs (29 indications) were available for the analysis, and again, there was no correlation with prices (R2=0.004 and 0.006, respectively).
Conclusions and relevance: Our results suggest that the prices of anticancer drugs in Italy do not reflect their therapeutic benefit. Drug price negotiations, which is mandatory by law in Italy, do not seem to ensure that prices correlate with clinical benefits provided by the cancer drugs. These results call for further efforts to establish the standard determinants of drug prices available at the time of negotiation. These findings need to be confirmed in other countries where price negotiations are in place. Moreover, further investigations may verify whether outcome data obtained after drug marketing would improve the correlation between prices and therapeutic benefit.
Keywords: antineoplastic agents; antineoplastic agents/therapeutic use; drug costs; treatment outcome.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: FP has received personal fees unrelated to the work presented in this article from Bayer, Ipsen, Astra Zeneca, Bristol Myers Squibb, Sandoz, Incyte, Celgene, Pierre Fabre, Janssen-Cilag.
Figures

References
-
- Quintiles IMS Institute Outlook for global medicines through 2021, 2016. Available: http://www.imshealth.com/en/thought-leadership/quintilesims-institute/re...
-
- European Commission - Press release Antitrust: Commission opens formal investigation into aspen pharma's pricing practices for cancer medicines. Brussels, 2017. Available: http://europa.eu/rapid/press-release_IP-17-1323_en.htm
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
