Impaired hemostatic activity of healthy transfused platelets in inherited and acquired platelet disorders: Mechanisms and implications
- PMID: 31826978
- PMCID: PMC10824274
- DOI: 10.1126/scitranslmed.aay0203
Impaired hemostatic activity of healthy transfused platelets in inherited and acquired platelet disorders: Mechanisms and implications
Abstract
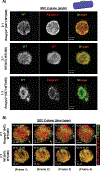
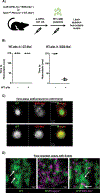
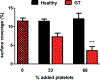
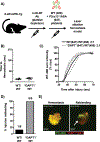
Platelet transfusions can fail to prevent bleeding in patients with inherited platelet function disorders (IPDs), such as Glanzmann's thrombasthenia (GT; integrin αIIbβ3 dysfunction), Bernard-Soulier syndrome [BSS; glycoprotein (GP) Ib/V/IX dysfunction], and the more recently identified nonsyndromic RASGRP2 variants. Here, we used IPD mouse models and real-time imaging of hemostatic plug formation to investigate whether dysfunctional platelets impair the hemostatic function of healthy donor [wild-type (WT)] platelets. In Rasgrp2-/- mice or mice with platelet-specific deficiency in the integrin adaptor protein TALIN1 ("GT-like"), WT platelet transfusion was ineffective unless the ratio between mutant and WT platelets was ~2:1. In contrast, thrombocytopenic mice or mice lacking the extracellular domain of GPIbα ("BSS-like") required very few transfused WT platelets to normalize hemostasis. Both Rasgrp2-/- and GT-like, but not BSS-like, platelets effectively localized to the injury site. Mechanistic studies identified at least two mechanisms of interference by dysfunctional platelets in IPDs: (i) delayed adhesion of WT donor platelets due to reduced access to GPIbα ligands exposed at sites of vascular injury and (ii) impaired consolidation of the hemostatic plug. We also investigated the hemostatic activity of transfused platelets in the setting of dual antiplatelet therapy (DAPT), an acquired platelet function disorder (APD). "DAPT" platelets did not prolong the time to initial hemostasis, but plugs were unstable and frequent rebleeding was observed. Thus, we propose that the endogenous platelet count and the ratio of transfused versus endogenous platelets should be considered when treating select IPD and APD patients with platelet transfusions.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


References
-
- Savage B, Saldívar E, Ruggeri ZM, Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor, Cell 84, 289–297 (1996). - PubMed
-
- Stegner D, Nieswandt B, Platelet receptor signaling in thrombus formation, J. Mol. Med 89, 109–121 (2011). - PubMed
-
- Lee RH, Stefanini L, Bergmeier W, in Platelets, Michelson AD, Ed. (Elsevier, 2019), pp. 329–348.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

