Differential remodeling of the electron transport chain is required to support TLR3 and TLR4 signaling and cytokine production in macrophages
- PMID: 31827178
- PMCID: PMC6906364
- DOI: 10.1038/s41598-019-55295-4
Differential remodeling of the electron transport chain is required to support TLR3 and TLR4 signaling and cytokine production in macrophages
Abstract
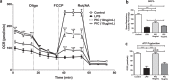
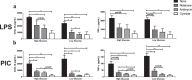
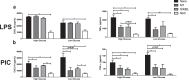
Increasing evidence suggests that mitochondria play a critical role in driving innate immune responses against bacteria and viruses. However, it is unclear if differential reprogramming of mitochondrial function contributes to the fine tuning of pathogen specific immune responses. Here, we found that TLR3 and TLR4 engagement on murine bone marrow derived macrophages was associated with differential remodeling of electron transport chain complex expression. This remodeling was associated with differential accumulation of mitochondrial and cytosolic ROS, which were required to support ligand specific inflammatory and antiviral cytokine production. We also found that the magnitude of TLR3, but not TLR4, responses were modulated by glucose availability. Under conditions of low glucose, TLR3 engagement was associated with increased ETC complex III expression, increased mitochondrial and cytosolic ROS and increased inflammatory and antiviral cytokine production. This amplification was selectively reversed by targeting superoxide production from the outer Q-binding site of the ETC complex III. These results suggest that ligand specific modulation of the ETC may act as a rheostat that fine tunes innate immune responses via mitochondrial ROS production. Modulation of these processes may represent a novel mechanism to modulate the nature as well as the magnitude of antiviral vs. inflammatory immune responses.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

