Biodistribution, Clearance And Morphological Alterations Of Intravenously Administered Iron Oxide Nanoparticles In Male Wistar Rats
- PMID: 31827324
- PMCID: PMC6902883
- DOI: 10.2147/IJN.S223142
Biodistribution, Clearance And Morphological Alterations Of Intravenously Administered Iron Oxide Nanoparticles In Male Wistar Rats
Abstract
Introduction: Nanoparticles are used worldwide because of their unique properties, with large-scale application in various fields, such as medicine, cosmetics and industries. In view of their widespread use, the potential adverse effects of nanoparticles have become a significant cause for concern, in terms of not only human health and safety but also the environment. The present investigation focused on establishing the bioaccumulation patterns and ultrastructural changes induced by retained iron oxide nanoparticles (IONPs) in various target organs of rats.
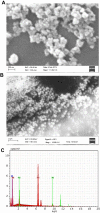
Methods: Twenty-four male Wistar rats were randomly divided into four groups. Experimental animals were intravenously administered different doses of IONPs (7.5 mg/kg, 15 mg/kg and 30 mg/kg) once in a week for 4 weeks. Urine and feces samples were collected on a daily basis to assess nanoparticle clearance and analyzed via atomic absorption spectroscopy (AAS). At the end of the experiment, rats were euthanized and different organs, including spleen, liver, kidney, lung, heart, testis and brain, were dissected. Bioaccumulation of iron in organs and ultrastructural changes induced by IONPs were determined.
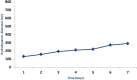
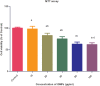
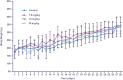
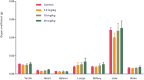
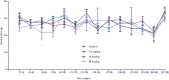
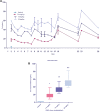
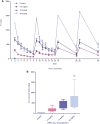
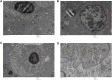
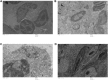
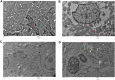
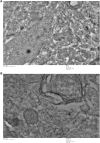
Results: The maximal concentration of iron was detected in spleen and minimal concentration in the brain. The level of iron accumulation in organs was as follows: spleen>blood>liver>kidney>lung>heart>testis>brain. The excretion profile in urine revealed maximum excretion on the day following administration that was maintained until day 28, whereas the iron content in feces remained high during the first three days after injection. A similar pattern was observed throughout the duration of the experiment. Ultrastructural alterations were detected in spleen, kidney, lung, heart, testis, brain and liver, indicative of cellular damage induced by accumulating nanoparticles in these organs.
Conclusion: Intravenous administration of IONPs results in ultrastructural changes and dose-dependent bioaccumulation in different organs of rats.
Keywords: bioaccumulation; metabolic cages; metal oxide nanoparticles; toxicity; ultrastructural changes.
© 2019 Gaharwar et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

