A Three-Dimensional Culture Model of Reversibly Quiescent Myogenic Cells
- PMID: 31827532
- PMCID: PMC6885280
- DOI: 10.1155/2019/7548160
A Three-Dimensional Culture Model of Reversibly Quiescent Myogenic Cells
Abstract
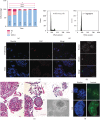
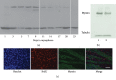
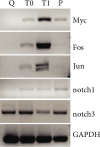
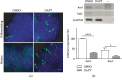
Satellite cells (SC) are the stem cells of skeletal muscles. They are quiescent in adult animals but resume proliferation to allow muscle hypertrophy or regeneration after injury. The mechanisms balancing quiescence, self-renewal, and differentiation of SC are difficult to analyze in vivo owing to their complexity and in vitro because the staminal character of SC is lost when they are removed from the niche and is not adequately reproduced in the culture models currently available. To overcome these difficulties, we set up a culture model of the myogenic C2C12 cell line in suspension. When C2C12 cells are cultured in suspension, they enter a state of quiescence and form three-dimensional aggregates (myospheres) that produce the extracellular matrix and express markers of quiescent SC. In the initial phase of culture, a portion of the cells fuses in syncytia and abandons the myospheres. The remaining cells are mononucleated and quiescent but resume proliferation and differentiation when plated in a monolayer. The notch pathway controls the quiescent state of the cells as shown by the fact that its inhibition leads to the resumption of differentiation. Within this context, notch3 appears to play a central role in the activity of this pathway since the expression of notch1 declines soon after aggregation. In summary, the culture model of C2C12 in suspension may be used to study the cellular interactions of muscle stem cells and the pathways controlling SC quiescence entrance and maintenance.
Copyright © 2019 Salvatore Aguanno et al.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

