Enhancement of Quercetin-Induced Apoptosis by Cotreatment with Autophagy Inhibitor Is Associated with Augmentation of BAK-Dependent Mitochondrial Pathway in Jurkat T Cells
- PMID: 31827702
- PMCID: PMC6885204
- DOI: 10.1155/2019/7989276
Enhancement of Quercetin-Induced Apoptosis by Cotreatment with Autophagy Inhibitor Is Associated with Augmentation of BAK-Dependent Mitochondrial Pathway in Jurkat T Cells
Abstract
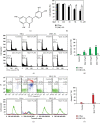
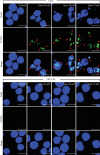
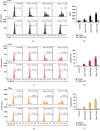
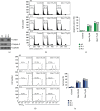
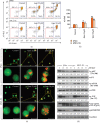
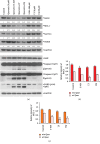
A flavonoid antioxidant quercetin promotes dose-dependent activation of the ATM-CHK-p53 pathway, downregulation of antiapoptotic survivin, and upregulation of proapoptotic NOXA in human T cell acute lymphoblastic leukemia Jurkat clones (J/Neo and J/BCL-XL). However, the downregulation of antiapoptotic BAG3 and MCL-1 occurred in J/Neo cells but not in J/BCL-XL cells overexpressing BCL-XL. Additionally, several BCL-XL-sensitive intrinsic mitochondrial apoptotic events including apoptotic sub-G1 cell accumulation, TUNEL-positive DNA fragmentation, BAK activation, mitochondrial membrane potential (Δψm) loss, caspase-9/caspase-8/caspase-3 activation, and PARP cleavage were induced only in J/Neo cells. Both cytosolic and mitochondrial ROS levels were elevated in quercetin-treated J/Neo cells; however, the ROS elevations were almost completely abrogated in J/BCL-XL cells, suggesting the ROS elevations were downstream of BCL-XL-sensitive mitochondrial damage and dysfunction. Wild-type A3, FADD-deficient I2.1, and caspase-8-deficient I9.2 Jurkat clones exhibited similar susceptibilities to the cytotoxicity of quercetin, excluding an involvement of extrinsic pathway in triggering the apoptosis. The autophagic events such as attenuation of AKT-mTOR pathway, formation of acridine orange-stainable acidic vesicular organelles, conversion of microtubule-associated protein 1 light chain 3-I (LC3-I) to LC3-II, and downregulation of p62/SQSTM1 level were detected in quercetin-treated J/Neo and J/BCL-XL cells, regardless of BCL-XL overexpression. Cotreatment with the autophagy inhibitor (3-methyladenine, LY294002, or chloroquine) resulted in a significant enhancement of quercetin-induced BAK activation and subsequently the mitochondrial damage-mediated apoptosis pathway by augmenting the downregulation of BAG3 and MCL-1 levels in J/Neo cells. These results demonstrated that quercetin induces intrinsic apoptosis and cytoprotective autophagy, and autophagy inhibition can potentiate BAK-dependent apoptotic activity of quercetin in Jurkat T cells.
Copyright © 2019 Eun Ji Ha et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

