Construction of a vascularized bladder with autologous adipose-derived stromal vascular fraction cells combined with bladder acellular matrix via tissue engineering
- PMID: 31827758
- PMCID: PMC6886281
- DOI: 10.1177/2041731419891256
Construction of a vascularized bladder with autologous adipose-derived stromal vascular fraction cells combined with bladder acellular matrix via tissue engineering
Abstract
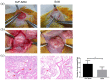
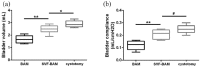
The formation of an effective vascular network can promote peripheral angiogenesis, ensuring an effective supply of blood, oxygen, and nutrients to an engineered bladder, which is important for bladder tissue engineering. Stromal vascular fraction cells (SVFs) promote vascularization and improve the function of injured tissues. In this study, adipose tissue-derived SVFs were introduced as an angiogenic cell source and seeded into the bladder acellular matrix (BAM) to generate a SVF-BAM complex for bladder reconstruction. The morphological regeneration and functional restoration of the engineered bladder were evaluated. In addition, we also explored the role of the Wnt5a/sFlt-1 noncanonical Wnt signaling pathway in regulating the angiogenesis of SVFs, and in maintaining the rational capability of SVFs to differentiate into vasculature in regenerated tissues. Histological assessment indicated that the SVF-BAM complex was more effective in promoting smooth muscle, vascular, and nerve regeneration than BAM alone and subsequently led to the restoration of bladder volume and bladder compliance. Moreover, exogenous Wnt5a was able to enhance angiogenesis by increasing the activity of MMP2, MMP9, and VEGFR2. Simultaneously, the expression of sFlt-1 was also increased, which enhanced the stability of the SVFs angiogenic capability. SVFs may be a potential cell source for tissue-engineered bladders. The Wnt5a/sFlt-1 pathway is involved in the regulation of autologous vascular formation by SVFs. The rational regulation of this pathway can promote neo-microvascularization in tissue-engineered bladders.
Keywords: Bladder augmentation; Wnt5a; bladder acellular matrix; stromal vascular fraction cells.
© The Author(s) 2019.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






References
-
- Zhou L, Yang B, Sun C, et al. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model. Tissue Eng Part A 2013; 19(1–2): 264–276. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

