Signaling pathways involved in colorectal cancer progression
- PMID: 31827763
- PMCID: PMC6889432
- DOI: 10.1186/s13578-019-0361-4
Signaling pathways involved in colorectal cancer progression
Abstract
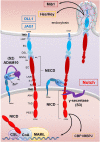
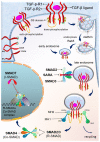
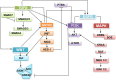
Colorectal cancer (CRC) is the fourth leading cause of the worldwide cancer mortality. Different molecular mechanisms have been attributed to the development and progress of CRC. In this review, we will focus on the mitogen-activated protein kinase (MAPK) cascades downstream of the epidermal growth factor receptor (EGFR), Notch, PI3K/AKT pathway, transforming growth factor-β (TGF-β), and Wnt signaling pathways. Various mutations in the components of these signaling pathways have been linked to the development of CRC. Accordingly, numerous efforts have been carried out to target the signaling pathways to develop novel therapeutic approaches. Herein, we review the signaling pathways involved in the incidence and progression of CRC, and the strategies for the therapy targeting components of signaling pathways in CRC.
Keywords: Colorectal cancer; EGFR; MAPK; Notch; TGF-β.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
-
- Tabana YM, Dahham SS, Shah AM, Majid A. Major signaling pathways of colorectal carcinogenesis. Recent Adv Colon Cancer. 2016;1:1–2. doi: 10.14302/issn.2471-7061.jcrc-14-579. - DOI

