In Vivo Models for the Study of Fibrosis
- PMID: 31827979
- PMCID: PMC6904938
- DOI: 10.1089/wound.2018.0909
In Vivo Models for the Study of Fibrosis
Abstract
Significance: Fibrosis and scar formation pose a substantial physiological and psychological burden on patients and a significant public health burden on the economy, estimated to be up to $12 billion a year. Fibrosis research is heavily reliant on in vivo models, but variations in animal models and differences between animal and human fibrosis necessitates careful selection of animal models to study fibrosis. There is also an increased need for improved animal models that recapitulate human pathophysiology. Recent Advances: Several murine and porcine models, including xenograft, drug-induced fibrosis, and mechanical load-induced fibrosis, for different types of fibrotic disease have been described in the literature. Recent findings have underscored the importance of mechanical forces in the pathophysiology of scarring. Critical Issues: Differences in skin, properties of subcutaneous tissue, and modes of fibrotic healing in animal models and humans provide challenges toward investigating fibrosis with in vivo models. While porcine models are typically better suited to study cutaneous fibrosis, murine models are preferred because of the ease of handling and availability of transgenic strains. Future Directions: There is a critical need to develop novel murine models that recapitulate the mechanical cues influencing fibrosis in humans, significantly increasing the translational value of fibrosis research. We advocate a translational pipeline that begins in mouse models with modified biomechanical environments for foundational molecular and cellular research before validation in porcine models that closely mimic the human condition.
Keywords: animal model; burn; fibrosis; foreign body reaction; hypertrophic scar; scar.
Copyright 2019, Mary Ann Liebert, Inc., publishers.
Figures

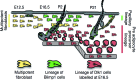



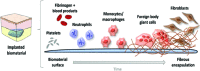
References
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 - PubMed
-
- Chandorkar YKR, Basu B. The Foreign Body Response Demystified. ACS Biomater Sci Eng 2019;5:19–44 - PubMed
-
- Aarabi S, Bhatt KA, Shi Y, et al. . Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 2007;21:3250–3261 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

