Gut microbiome and CAR-T therapy
- PMID: 31827982
- PMCID: PMC6862813
- DOI: 10.1186/s40164-019-0155-8
Gut microbiome and CAR-T therapy
Abstract
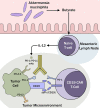
Considerable progress has been made in cancer therapeutics recently with targeted strategies that are efficacious and less toxic. Immunotherapy and chimeric antigen receptor (CAR) T-cells are increasingly being evaluated in a variety of tumors in the relapsed/refractory as well as frontline disease settings, predominantly in hematologic malignancies (HM). Despite impressive outcomes in select patients, there remains significant heterogeneity in clinical response to CAR T-cells. The gut microbiome has emerged as one of the key host factors that could potentially be modulated to enhance responses to immunotherapy. Several recent human studies receiving immunotherapy showed a significantly superior response and survival in patients with the more diverse gut microbiome. Currently, it is unknown if gut microbiota modulates anti-tumor responses to CAR T-cells. Based on molecular and immunological understanding, we hypothesize that strategically manipulating gut microbiota may enhance responses to CAR T-cells. In this review, we further discuss resistance mechanisms to CAR T-cells in HM, potential approaches to overcome resistance by harnessing gut microbiota and other related novel strategies.
Keywords: CAR T-cells; CRISPR/cas9; Dysbiosis; Gut microbiome; Immuno-oncology; Immunotherapy; TRUCKs.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

