Development of Reporting Guidelines for Animal Health Surveillance-AHSURED
- PMID: 31828080
- PMCID: PMC6890601
- DOI: 10.3389/fvets.2019.00426
Development of Reporting Guidelines for Animal Health Surveillance-AHSURED
Abstract
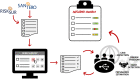
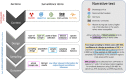
With the current trend in animal health surveillance toward risk-based designs and a gradual transition to output-based standards, greater flexibility in surveillance design is both required and allowed. However, the increase in flexibility requires more transparency regarding surveillance, its activities, design and implementation. Such transparency allows stakeholders, trade partners, decision-makers and risk assessors to accurately interpret the validity of the surveillance outcomes. This paper presents the first version of the Animal Health Surveillance Reporting Guidelines (AHSURED) and the process by which they have been developed. The goal of AHSURED was to produce a set of reporting guidelines that supports communication of surveillance activities in the form of narrative descriptions. Reporting guidelines come from the field of evidence-based medicine and their aim is to improve consistency and quality of information reported in scientific journals. They usually consist of a checklist of items to be reported, a description/definition of each item, and an explanation and elaboration document. Examples of well-reported items are frequently provided. Additionally, it is common to make available a website where the guidelines are documented and maintained. This first version of the AHSURED guidelines consists of a checklist of 40 items organized in 11 sections (i.e., surveillance system building blocks), which is available as a wiki at https://github.com/SVA-SE/AHSURED/wiki. The choice of a wiki format will allow for further inputs from surveillance experts who were not involved in the earlier stages of development. This will promote an up-to-date refined guideline document.
Keywords: animal health surveillance; expert elicitation; harmonization; output-based standards; reporting guidelines.
Copyright © 2019 Comin, Grewar, Schaik, Schwermer, Paré, El Allaki, Drewe, Lopes Antunes, Estberg, Horan, Calvo-Artavia, Jibril, Martínez-Avilés, Van der Stede, Antoniou and Lindberg.
Figures
References
-
- OIE—World Organisation for Animal Health Animal Health Surveillance in Terrestrial Animal Health Code, 28th Edn Paris: OIE; (2019). Chapter 1.4; p. 10.
-
- Schauer B, Martínez-Avilés M, Comin A, Rodríguez Prieto V, Dórea F, Häsler B, et al. “Surveillance is a public good” - but how public is it? In: 8th Annual Meeting EPIZONE 23 - 25 September. Copenhagen: (2014). Available online at: http://www.fp7-risksur.eu/sites/default/files/documents/publications/Epi... (Accessed July 11, 2019).
-
- Comin A, Häsler B, Hoinville L, Peyre M, Dórea F, Schauer B, et al. RISKSUR tools: taking animal health surveillance into the future through interdisciplinary integration of scientific evidence. In: Proceedings of the Annual meeting of the Society of Veterinary Epidemiology and Preventive Medicine. Elsinore: (2016).
LinkOut - more resources
Full Text Sources

