Enzymatic promiscuity and the evolution of bioluminescence
- PMID: 31828943
- PMCID: PMC7217382
- DOI: 10.1111/febs.15176
Enzymatic promiscuity and the evolution of bioluminescence
Abstract
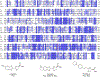
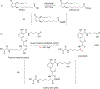
Bioluminescence occurs when an enzyme, known as a luciferase, oxidizes a small-molecule substrate, known as a luciferin. Nature has evolved multiple distinct luciferases and luciferins independently, all of which accomplish the impressive feat of light emission. One of the best-known examples of bioluminescence is exhibited by fireflies, a class of beetles that use d-luciferin as their substrate. The evolution of bioluminescence in beetles is thought to have emerged from ancestral fatty acyl-CoA synthetase (ACS) enzymes present in all insects. This theory is supported by multiple lines of evidence: Beetle luciferases share high sequence identity with these enzymes, often retain ACS activity, and some ACS enzymes from nonluminous insects can catalyze bioluminescence from synthetic d-luciferin analogues. Recent sequencing of firefly genomes and transcriptomes further illuminates how the duplication of ACS enzymes and subsequent diversification drove the evolution of bioluminescence. These genetic analyses have also uncovered candidate enzymes that may participate in luciferin metabolism. With the publication of the genomes and transcriptomes of fireflies and related insects, we are now better positioned to dissect and learn from the evolution of bioluminescence in beetles.
Keywords: adenylation; beetles; bioluminescence; chemistry; enzymes; evolution; firefly; luciferase; luciferin; metabolism.
© 2019 Federation of European Biochemical Societies.
Conflict of interest statement
Figures
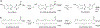




References
-
- Fallon TR, Lower SE, Chang C- H, Bessho-Uehara M, Martin GJ, Bewick AJ, Behringer M, Debat HJ, Wong I, Day JC, Suvorov A, Silva CJ, Stanger-Hall KF, Hall DW, Schmitz RJ, Nelson DR, Lewis SM, Shigenobu S, Bybee SM, Larracuente AM, Oba Y & Weng J-K (2018) Firefly genomes illuminate parallel origins of bioluminescence in beetles. eLife 7, e36495. - PMC - PubMed
-
- Petushkov VN, Dubinnyi MA, Tsarkova AS, Rodionova NS, Baranov MS, Kublitski VS, Shimomura O & Yampolsky I V. (2014) A Novel Type of Luciferin from the Siberian Luminous Earthworm Fridericia heliota : Structure Elucidation by Spectral Studies and Total Synthesis. Angewandte Chemie International Edition 53, 5566–5568. - PubMed
-
- Kaskova ZM, Dörr FA, Petushkov VN, Purtov K V., Tsarkova AS, Rodionova NS, Mineev KS, Guglya EB, Kotlobay A, Baleeva NS, Baranov MS, Arseniev AS, Gitelson JI, Lukyanov S, Suzuki Y, Kanie S, Pinto E, Di Mascio P, Waldenmaier HE, Pereira TA, Carvalho RP, Oliveira AG, Oba Y, Bastos EL, Stevani CV & Yampolsky IV (2017) Mechanism and color modulation of fungal bioluminescence. Science Advances 3, e1602847. - PMC - PubMed
-
- Kaskova ZM, Tsarkova AS & Yampolsky I V. (2016) 1001 lights: luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chemical Society Reviews 45, 6048–6077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

