Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience
- PMID: 31829148
- PMCID: PMC6907137
- DOI: 10.1186/s12890-019-1010-8
Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience
Abstract
Background: The Robotic Endoscopic System (Auris Health, Inc., Redwood City, CA) has the potential to overcome several limitations of contemporary guided-bronchoscopic technologies for the diagnosis of lung lesions. Our objective is to report on the initial post-marketing feasibility, safety and diagnostic yield of this technology.
Methods: We retrospectively reviewed data on consecutive cases in which robot-assisted bronchoscopy was used to sample lung lesions at four centers in the US (academic and community) from June 15th, 2018 to December 15th, 2018.
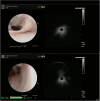
Results: One hundred and sixty-seven lesions in 165 patients were included in the analysis, with an average follow-up of 185 ± 55 days. The average size of target lesions was 25.0 ± 15.0 mm. Seventy-one percent were located in the peripheral third of the lung. Pneumothorax and airway bleeding occurred in 3.6 and 2.4% cases, respectively. Navigation was successful in 88.6% of cases. Tissue samples were successfully obtained in 98.8%. The diagnostic yield estimates ranged from 69.1 to 77% assuming the cases of biopsy-proven inflammation without any follow-up information (N = 13) were non-diagnostic and diagnostic, respectively. The yield was 81.5, 71.7 and 26.9% for concentric, eccentric and absent r-EBUS views, respectively. Diagnostic yield was not affected by lesion size, density, lobar location or centrality.
Conclusions: RAB implementation in community and academic centers is safe and feasible, with an initial diagnostic yield of 69.1-77% in patients with lung lesions that require diagnostic bronchoscopy. Comparative trials with the existing bronchoscopic technologies are needed to determine cost-effectiveness of this technology.
Keywords: Biopsy; Electromagnetic navigation; Lung cancer; Lung lesion; Robotic bronchoscopy.
Conflict of interest statement
Dr. Murgu has acted as a paid educational consultant for Olympus, Cook Inc., Pinnacle Biologics and Boston Scientific. Dr. Kovacs, Dr. Manley, Dr. Cumbo-Nacheli and Dr. Egan are consultants for Auris and have received consulting fees. Dr. Hogarth has received honoraria for preceptorships, lectures, and consulting from Boston Scientific. He has also received unrestricted education grants from Boston Scientific. He has received honorarium from Biodesix and Veracyte for lectures and consulting. He has received unrestricted education grants from Biodesix. He has been part of contracted research studies for Veracyte. He is a consultant for Auris and also a stock holder. Dr. Chaddha, Dr. Bhavani, Dr. Kumar and Dr. Shende have nothing to declare.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

